EXHIBIT 99.1
Published on June 4, 2021
Exhibit 99.2
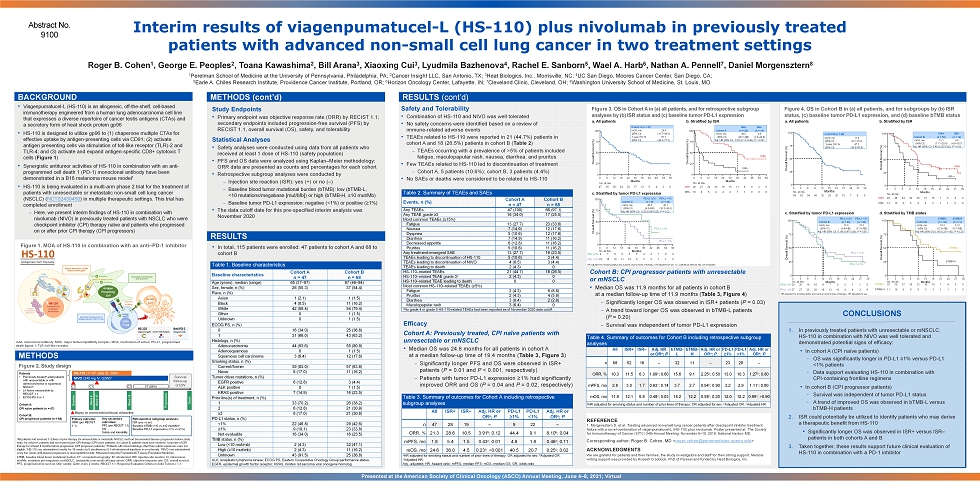
Interim results of viagenpumatucel - L (HS - 110) plus nivolumab in previously treated patients with advanced non - small cell lung cancer in two treatment settings BACKGROUND • Viagenpumatucel - L (HS - 110) is an allogeneic, off - the - shelf, cell - based immunotherapy engineered from a human lung adenocarcinoma cell line that expresses a diverse repertoire of cancer testis antigens (CTAs) and a secretory form of heat shock protein gp96 • HS - 110 is designed to utilize gp96 to (1) chaperone multiple CTAs for effective uptake by antigen - presenting cells via CD91; (2) activate antigen presenting cells via stimulation of toll - like receptor (TLR) - 2 and TLR - 4; and (3) activate and expand antigen - specific CD8+ cytotoxic T cells ( Figure 1 ) • Synergistic antitumor activities of HS - 110 in combination with an a nti - programmed cell death 1 (PD - 1) monoclonal antibody have been demonstrated in a B16 melanoma mouse model 1 • HS - 110 is being evaluated in a multi - arm phase 2 trial for the treatment of patients with unresectable or metastatic non - small cell lung cancer (NSCLC) ( NCT02439450 ) in multiple therapeutic settings. This trial has completed enrollment – Here, we present interim findings of HS - 110 in combination with nivolumab (NIVO) in previously treated patients with NSCLC who were checkpoint inhibitor (CPI) therapy naïve and patients who progressed on or after prior CPI therapy (CPI progressors) Abstract No. 9100 Roger B. Cohen 1 , George E. Peoples 2 , Toana Kawashima 2 , Bill Arana 3 , Xiaoxing Cui 3 , Lyudmila Bazhenova 4 , Rachel E. Sanborn 5 , Wael A. Harb 6 , Nathan A. Pennell 7 , Daniel Morgensztern 8 1 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; 2 Cancer Insight LLC, San Antonio, TX; 3 Heat Biologics, Inc., Morrisville, NC; 4 UC San Diego, Moores Cancer Center, San Diego, CA; 5 Earle A. Chiles Research Institute, Providence Cancer Institute, Portland, OR; 6 Horizon Oncology Center, Lafayette, IN; 7 Cleveland Clinic, Cleveland, OH; 8 Washington University School of Medicine, St. Louis, MO Figure 1 . MOA of HS - 110 in combination with an anti – PD - 1 inhibitor Study Endpoints • Primary endpoint was objective response rate (ORR) by RECIST 1.1; secondary endpoints included progression - free survival (PFS) by RECIST 1.1, overall survival (OS), safety, and tolerability Statistical Analyses • Safety analyses were conducted using data from all patients who received at least 1 dose of HS - 110 (safety population) • PFS and OS data were analyzed using Kaplan – Meier methodology; ORR data are presented as counts and percentages for each cohort • Retrospective subgroup analyses were conducted by – Injection site reaction (ISR): yes (+) or no ( – ) – Baseline blood tumor mutational burden (bTMB): low (bTMB - L, <10 mutations/megabase [mut/Mb]) or high (bTMB - H, ≥10 mut/Mb) – Baseline tumor PD - L1 expression: negative (<1%) or positive (≥1%) • The data cutoff date for this pre - specified interim analysis was November 2020 Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, June 4 – 8, 2021; Virtual REFERENCE 1 . Morgensztern D, et al. Treating advanced non - small lung cancer patients after checkpoint inhibitor treatment failure with a novel combination of viagenpumatucel - L (HS - 110) plus nivolumab. Poster presented at: The Society for Immunotherapy of Cancer (SITC) 34th Annual Meeting; November 6 – 10, 2019; National Harbor, MD. Corresponding author: Roger B. Cohen, MD < roger.cohen@pennmedicine.upenn.edu > ACKNOWLEDGMENTS We are grateful for patients and their families, the study investigators and staff for their strong support. Medical writing support was provided by Russell Craddock, PhD of Parexel and funded by Heat Biologics, Inc. RESULTS (cont’d) CONCLUSIONS 1. In previously treated patients with unresectable or mNSCLC, HS - 110 in combination with NIVO was well tolerated and demonstrated potential signs of efficacy: • In cohort A (CPI naïve patients) – OS was significantly longer in PD - L1 ≥1% versus PD - L1 <1% patients – Data support evaluating HS - 110 in combination with CPI - containing frontline regimens • In cohort B (CPI progressor patients) – Survival was independent of tumor PD - L1 status – A trend of improved OS was observed in bTMB - L versus bTMB - H patients 2. ISR could potentially be utilized to identify patients who may derive a therapeutic benefit from HS - 110 • Significantly longer OS was observed in ISR+ versus ISR – patients in both cohorts A and B 3. Taken together, these results support future clinical evaluation of HS - 110 in combination with a PD - 1 inhibitor mAb, monoclonal antibody; MHC, major histocompatibility complex; MOA, mechanism of action; PD - L1, programmed death ligand 1; TLR, toll - like receptor. RESULTS • In total, 115 patients were enrolled: 47 patients to cohort A and 68 to cohort B Table 1. Baseline characteristics Baseline characteristics Cohort A n = 47 Cohort B n = 68 Age (years), median (range) 65 (37 – 87) 67 (46 – 84) Sex, female, n (%) 26 (55.3) 37 (54.4) Race, n (%) Asian 1 (2.1) 1 (1.5) Black 4 (8.5) 11 (16.2) White 42 (89.4) 54 (79.4) Other 0 1 (1.5) Unknown 0 1 (1.5) ECOG PS, n (%) 0 16 (34.0) 25 (36.8) 1 31 (66.0) 43 (63.2) Histology, n (%) Adenocarcinoma 44 (93.6) 55 (80.9) Adenosquamous 0 1 (1.5) Squamous cell carcinoma 3 (6.4) 12 (17.6) Smoking status, n (%) Current/former 39 (83.0) 57 (83.8) Never 8 (17.0) 11 (16.2) Tumor driver mutations, n (%) EGFR positive 6 (12.8) 3 (4.4) ALK positive 0 1 (1.5) KRAS positive 7 (14.9) 16 (23.5) Prior line(s) of treatment, n (%) 1 33 (70.2) 26 (38.2) 2 6 (12.8) 21 (30.9) ≥3 8 (17.0) 21 (30.8) PD - L1 status, n (%) <1% 22 (46.8) 29 (42.6) ≥1% 9 (19.1) 23 (33.8) Not evaluable 16 (34.0) 16 (23.5) TMB status, n (%) Low (<10 mut/mb) 2 (4.3) 32 (47.1) High (≥10 mut/mb) 2 (4.3) 11 (16.2) Unknown 43 (91.5) 25 (36.8) ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog. Table 2. Summary of TEAEs and SAEs Events, n (%) Cohort A n = 47 Cohort B n = 68 Any TEAEs 47 (100) 66 (97.1) Any TEAE grade ≥3 16 (34.0) 17 (25.0) Most common TEAEs (≥15%) Fatigue 13 (27.7) 23 (33.8) Nausea 7 (14.9) 12 (17.6) Dyspnea 5 (10.6) 12 (17.6) Diarrhea 7 (14.9) 11 (16.2) Decreased appetite 6 (12.8) 11 (16.2) Pruritus 5 (10.6) 11 (16.2) Any treatment - emergent SAE 13 (27.7) 16 (23.5) TEAEs leading to discontinuation of HS - 110 5 (10.6) 3 (4.4) TEAEs leading to discontinuation of NIVO 4 (8.5) 3 (4.4) TEAEs leading to death 2 (4.3) 0 HS - 110 – related TEAEs 21 (44.7) 18 (26.5) HS - 110 – related TEAE grade 3 a 2 (4.3) 0 HS - 110 – related TEAE leading to death 0 0 Most common HS - 110 – related TEAEs (≥5%) Fatigue 2 (4.3) 6 (8.8) Pruritus 2 (4.3) 4 (5.9) Diarrhea 3 (6.4) 2 (2.9) Maculopapular rash 3 (6.4) 0 a No grade 4 or grade 5 HS - 110 - related TEAEs had been reported as of November 2020 data cutoff. Table 3. Summary of outcomes for Cohort A including retrospective subgroup analyses All ISR+ ISR – Adj. HR or OR a ; P PD - L1 ≥1% PD - L1 <1% Adj. HR or OR a ; P n 47 28 19 – 9 22 – ORR, % 21.3 28.6 10.5 3.91 b ; 0.12 44.4 9.1 8.10 b ; 0.04 mPFS, mo 1.8 5.4 1.5 0.43 c ; 0.01 4.8 1.8 0.46 c ; 0.11 mOS, mo 24.6 36.0 4.5 0.23 c ; <0.001 40.5 20.7 0.25 c ; 0.02 a HR adjusted for smoking status and number of prior lines of therapy; OR adjusted for sex. b Adjusted OR. c Adjusted HR. Adj., adjusted; HR, hazard ratio; mPFS, median PFS; mOS, median OS; OR, odds ratio. Table 4. Summary of outcomes for Cohort B including retrospective subgroup analyses All ISR+ ISR – Adj. HR or OR a ; P bTMB - L bTMB - H Adj. HR or OR a ; P PD - L1 ≥1% PD - L1 <1% Adj. HR or OR a ; P n 68 52 16 – 32 11 – 23 29 – ORR, % 10.3 11.5 6.3 1.99 b ; 0.60 15.6 9.1 2.25 b ; 0.50 13.0 10.3 1.27 b ; 0.80 mPFS, mo 2.8 3.0 1.7 0.63 c ; 0.14 3.7 2.7 0.94 c ; 0.90 3.2 2.9 1.11 c ; 0.80 mOS, mo 11.9 12.1 6.8 0.48 c ; 0.03 18.2 12.2 0.58 c ; 0.20 12.0 12.2 0.99 c ; >0.90 a HR adjusted for smoking status and number of prior lines of therapy; OR adjusted for sex. b Adjusted OR. c Adjusted HR. Safety and T olerability • Combination of HS - 110 and NIVO was well tolerated • No safety concerns were identified based on a review of immune - related adverse events • TEAEs related to HS - 110 were reported in 21 (44.7%) patients in cohort A and 18 (26.5%) patients in cohort B ( Table 2 ) – TEAEs occurring with a prevalence of >5% of patients included fatigue, maculopapular rash, nausea, diarrhea, and pruritus • Few TEAEs related to HS - 110 led to discontinuation of treatment – Cohort A, 5 patients (10.6%); cohort B, 3 patients (4.4%) • No SAEs or deaths were considered to be related to HS - 110 METHODS Figure 2 . Study design a All patients had received 1 – 3 lines of prior therapy for unresectable or metastatic NSCLC and had documented disease progression before study entry; for cohort A, patients had not received prior CPI therapy (CPI naïve patients); for cohort B, patients must have recei ved 1 prior line of CPI therapy for at least 4 months before progression (CPI progressor patients). b Patients with mixed histology other than adeno - squamous were not eligible. c HS - 110 was administered weekly for 18 weeks via 5 simultaneous 0.1 - ml intradermal injections in an extremity. d NIVO was administered every two weeks until disease progression or unacceptable toxicity. e Measured using the FoundationACT assay (Foundation Medicine). bTMB, baseline blood tumor mutational burden; CT, computed tomography; ID, intradermal; ISR, injection - site reaction; IV, intrav enous; mut/Mb, mutations per megabase; (m)NSCLC, (metastatic) non - small cell lung cancer; ORR, objective response rate; OS, overall sur vival; PFS, progression - free survival; QW, weekly; Q2W, every 2 weeks; RECIST 1.1, Response Evaluation Criteria in Solid Tumors v.1.1. Retrospective subgroup analyses: ISR (yes vs no) Baseline bTMB (<10 vs ≥10 mut/Mb) e Baseline PD - L1 expression (<1% vs ≥1%) HS - 110 (1 î 10 7 cells ID, QW) c NIVO (240 mg IV, Q2W) d CT Baseline Week 9 Week 18 Week 10 CT ( Q8W ) Progression Death Biopsy (or recent archival tissue) at baseline Survival follow - up Q12W CT CT Patients • Previously treated a adult patients with unresectable or with adenocarcinoma or squamous NSCLC b • ≥1 lesion measurable by RECIST 1.1 • ECOG PS 0 or 1 Cohort A: CPI naïve patients (n = 47) Cohort B: CPI progressor patients (n = 68) Primary outcome: ORR (per RECIST 1.1) METHODS (cont’d) Efficacy Cohort A: Previously treated, CPI naïve patients with unresectable or mNSCLC • Median OS was 24.6 months for all patients in cohort A at a median follow - up time of 19.4 months ( Table 3, Figure 3 ) – Significantly longer PFS and OS were observed in ISR+ patients ( P = 0.01 and P < 0.001, respectively) – Patients with tumor PD - L1 expression ≥1% had significantly improved ORR and OS ( P = 0.04 and P = 0.02, respectively) Cohort B: CPI progressor patients with unresectable or mNSCLC • Median OS was 11.9 months for all patients in cohort B at a median follow - up time of 11.9 months ( Table 3, Figure 4 ) – Significantly longer OS was observed in ISR+ patients ( P = 0.03) – A trend toward longer OS was observed in bTMB - L patients ( P = 0.20) – Survival was independent of tumor PD - L1 expression Key secondary outcomes: PFS (per RECIST 1.1) OS Safety and tolerability HR adjusted for smoking status and number of prior lines of therapy; OR adjusted for sex. HR adjusted for smoking status and number of prior lines of therapy; OR adjusted for sex. CI, confidence interval; NE, not ev alu able. 0 25 50 75 100 0 6 12 18 24 30 36 42 48 54 60 Months Overall Survival (%) 0 25 50 75 100 0 6 12 18 24 30 36 42 48 54 60 Months Overall Survival (%) 0 25 50 75 100 0 6 12 18 24 30 36 42 48 54 60 Months Overall Survival (%) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | PD - L1 <1% a. All patients c. Stratified by tumor PD - L1 expression b. Stratified by ISR ISR – ISR+ 0 25 50 75 100 0 3 6 9 12 15 18 21 24 27 30 Months Overall Survival (%) PD - L1 <1% 0 25 50 75 100 0 6 9 15 21 27 3 12 18 24 30 Months Overall Survival (%) | | | | | | | | | | | | | | | | | | | | | | | | | | | | a. All patients c. Stratified by tumor PD - L1 expression d. Stratified by TMB status b. Stratified by ISR 47 36 29 24 23 17 12 9 4 2 0 No. at risk 28 27 24 20 19 15 11 8 4 2 0 19 9 5 4 4 2 1 1 0 0 0 No. at risk ISR+ ISR – 9 7 6 6 6 5 4 3 1 0 0 22 16 14 11 10 6 3 2 1 1 0 No. at risk PD - L1 ≥1% PD - L1 <1% PD - L1 ≥1% 68 62 54 45 32 25 20 10 7 5 0 No. at risk 49 48 44 39 28 21 16 7 5 4 0 13 13 9 5 4 3 2 1 1 0 0 0 25 50 75 100 0 3 6 9 12 15 18 21 24 27 30 Months Overall Survival (%) ISR — ISR+ | | | | | | | | | | | | | | | No. at risk ISR+ ISR – PD - L1 ≥1% PD - L1 ≥1% PD - L1 <1% 20 20 18 16 11 7 5 3 1 1 0 27 27 25 20 16 12 10 4 4 3 0 32 30 27 26 21 18 15 7 4 3 0 11 10 9 8 6 3 3 1 1 1 0 bTMB - L bTMB - H 0 25 50 75 100 0 6 9 15 21 27 3 12 18 24 30 Months Overall Survival (%) bTMB - L bTMB - H | | | | | | | | | | | | | | No. at risk No. at risk Cohort A ISR+ (n = 28) ISR – (n = 19) mOS, mo 36.0 4.5 (95% CI) (28.7 – NE) (1.6 – 24.6) Adj. HR (95% CI) 0.23 (0.11 – 0.49), P < 0.001 Figure 3 . OS in Cohort A in (a) all patients, and for retrospective subgroup analyses by (b) ISR status and (c) baseline tumor PD - L1 expression Figure 4. OS in Cohort B in (a) all patients, and for subgroups by (b) ISR status, (c) baseline tumor PD - L1 expression, and (d) baseline bTMB status Cohort A PD - L1 ≥1% (n = 9) PD - L1 <1% (n = 22) mOS, mo 40.5 20.7 (95% CI) (8.0 – NE) (10.2 – 36.0) Adj. HR (95% CI) 0.25 (0.08 – 0.82), P = 0.02 Cohort B ISR+ (n = 52) ISR – (n = 16) mOS, mo 12.1 6.8 (95% CI) (11.1 – 20.8) (4.9 – 19.7) Adj. HR (95% CI) 0.48 (0.25 – 0.92), P = 0.03 Cohort B PD - L1 ≥1% (n = 23) PD - L1 <1% (n = 29) mOS, mo 12.0 12.2 (95% CI) (9.4 – NE) (11.5 – NE) Adj. HR (95% CI) 0. 99 (0.47 – 2.09), P = 0.90 Cohort B bTMB - L (n = 32) bTMB - H (n = 11) mOS, mo 18.2 12.2 (95% CI) (12.9 – NE) (9.7 – NE) Adj. HR (95% CI) 0. 58 (0.23 – 1.42), P = 0.20 Cohort A ( n = 47) mOS, mo 24.6 (95% CI) (11.7 – 36.0) 1 - year OS, % 61.7 (95% CI) (49.3 – 77.3) Cohort B ( n = 68) mOS, mo 11.9 (95% CI) (9.7 – 16.3) 1 - year OS, % 47.1 (95% CI) (36.6 – 60.6) HS - 110 A llogeneic Cell Therapy