EXHIBIT 99.2
Published on April 20, 2022

N IGHT H AWK B IOSCIENCES CORPORATE PRESENTATION April 2022 PLEASE NOTE: Heat Biologics, Inc. anticipates transitioning to NightHawk Biosciences, Inc effective May 3, 2022.

This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Priva te Securities Litigation Reform Act of 1995, as amended. In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “ app roximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, an alyses or current expectations concerning, among other things, the timing of the opening of our facilities in San Antonino, Texas and Manhattan , K ansas, our ongoing and planned discovery and development of drugs targeting cancer, non - oncology, infectious disease medical countermeasures, our plann ed biosecurity/biodefense initiative, our planned bioanalytics , process development and manufacturing activities, our biologics drug discovery, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to pa rtn er our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regar din g clinical trial data, our results of operations, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or ma y o ccur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward - looking statement conta ined in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual result s of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the for war d - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of o ur Annual Report on Form 10 - K for the year ended December 31, 2021, our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent fi lings with the Securities and Exchange Commission (collectively, our “SEC Filings”). In addition, even if our results of operations, financi al condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this present ation, they may not be predictive of results or developments in future periods. Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of th is presentation, except as required by law. 2 Forward Looking Statements

3 Snapshot of N IGHT H AWK Fully integrated biopharmaceutical company developing novel therapies that arm the immune system • Key programs targeting oncology, inflammation, and medical countermeasures • Well - capitalized with strong balance sheet NightHawk Bio’s ecosystem enables agility to innovate and pivot • Ecosystem of integrated companies with efficient control of discovery, preclinical/clinical development, and biomanufacturing • End - to - end development from bench to commercial Major programs include: • Anthim® ( obiltoxaximab ) – FDA approved best - in - class antitoxin treatment for inhalational anthrax • HS - 110 – “off - the - shelf” cell - based immunotherapy with positive results in NSCLC (Phase 2) • PTX - 35 – potential first - in - class immunomodulatory antibody for treatment of solid tumors (Phase 1) • RapidVax ® – novel vaccine platform designed to a ccelerate time to clinic to combat emerging biological threats

4 Discovery N IGHT H AWK Ecosystem Streamlining Innovative Discoveries to Accelerate Clinical Development Preclinical/Clinical Development Biomanufacturing

Skunkworx Bio Discovery Sciences “Biology Drives Innovation” • Primary proprietary platform designed to enable rapid selection and validation of novel therapeutics for preclinical development • Additional novel therapeutic targeting and drug delivery platforms under development Unique Hotspot approach based on Pocket Biologics • Novel , highly diverse, proprietary compound libraries used to identify small proteins and human antibodies which bind to critical druggable targets • Advanced computational methods and bioinformatics inform target identification and improve development of candidate therapeutics Located in the New Jersey Bioscience Center - North Brunswick, NJ • Highly selective bioscience incubator in the heart of New Jersey’s “Research Corridor” 5
Oncology and Inflammation Development Heat Biologics – Realizing Immune Potential • Developing first - in - class immunomodulators that realize the potential of the immune system to treat and protect against a wide range of diseases • Proprietary gp96 - based vaccines to re - stimulate the immune system’s anti - tumor response • Phase 2 candidate HS - 110 ( vigenpumatucel - L) is an off - the - shelf allogenic cell therapy designed to stimulate immune responses against NSCLC Pelican Therapeutics – Next Generation T cell Immunotherapy for Cancer • Lead candidate, the monoclonal antibody PTX - 35, is an agonist of TNFRSF25 with the potential to shift the balance between inflammation and immunosuppression • In Phase I solid tumors as a potential first - in - class T cell co - stimulator • $15.2 million Cancer Prevention Institute of Texas (CPRIT) grant (the “CPRIT Grant”) to support development of PTX - 35 6
Elusys Therapeutics Medical Countermeasures Definitive agreement for acquisition executed in December 2021 • Sophisticated knowledge and hands - on experience in biodefense biologics • Program Management expertise with government agencies including the NIH, DoD, and BARDA Developer and marketer of Anthim ® , best - in - class monoclonal antibody antitoxin for anthrax • For treatment of inhalation anthrax in combination with antibiotics or, and as a prophylaxis when alternative therapies are not available or are not appropriate. Prescribing information: https://anthim.com/ • FDA approval in 2016, orphan drug designation • Approved in 2020 as only licensed anthrax treatment in EU, UK, and CA • Orphan drug designation in EU at time of approval Established government partnerships and funding • Received over $250M of non - dilutive development contracts from the NIH, DoD, and BARDA • Completed $70M in procurement contracts to supply Anthim to the U.S. Strategic National Stockpile (2016, 2018) • Completed first phase of contract for $50 million; HHS options to procure up to $31 million of Anthim by the first half of 20 23 7

Scorpion Biological Services Biomanufacturing and Analytical Labs Designed to provide scale - up GMP process development and biomanufacturing, cell and immuno - assay development, and bioanalytical lab services • Dedicated capacity to accelerate NightHawk preclinical and clinical development efforts • Contract manufacturing and bioanalytical services for external biopharmaceutical companies • Focus on American supply chain for materials and equipment Clinical Scale Facility, San Antonio, Texas – grand opening Q3 2022* • Designed to provide a scalable process development • Production of GMP material to large - scale clinical manufacturing and cold storage facilities Commercial Scale Facility, Manhattan, Kansas – breaking ground Q3 2022* • 500,000 sq. ft. state - of - the - art cGMP commercial biomanufacturing facility • 48+ bioreactors, ~144,000 liters for biomanufacturing of large - scale biologics 8 * per scheduled timeline
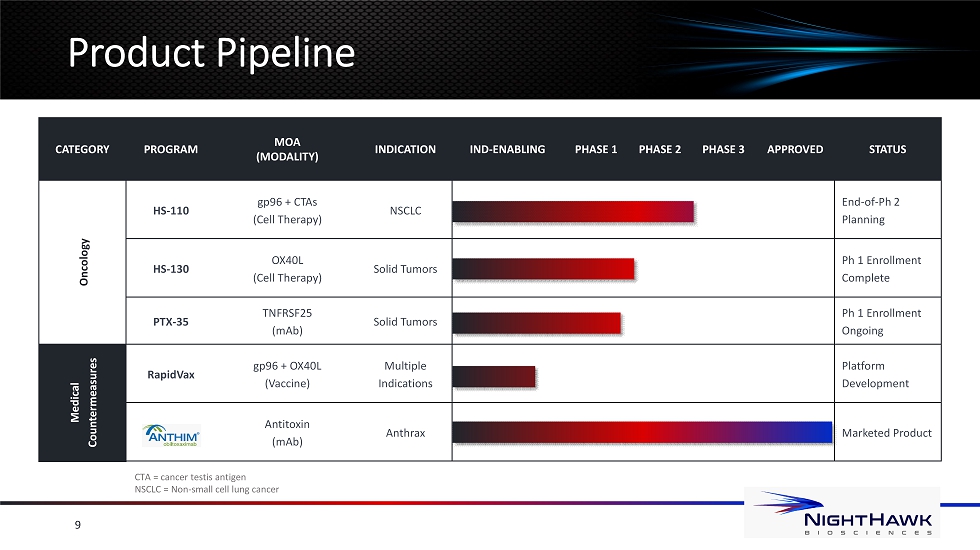
P roduct Pipeline 9 CTA = cancer testis antigen NSCLC = Non - small cell lung cancer CATEGORY PROGRAM MOA (MODALITY) INDICATION IND - ENABLING PHASE 1 PHASE 2 PHASE 3 APPROVED STATUS Oncology HS - 110 gp96 + CTAs (Cell Therapy) NSCLC End - of - Ph 2 Planning HS - 130 OX40L (Cell Therapy) Solid Tumors Ph 1 Enrollment Complete PTX - 35 TNFRSF25 (mAb) Solid Tumors Ph 1 Enrollment Ongoing Medical Countermeasures RapidVax gp96 + OX40L (Vaccine) Multiple Indications Platform Development Antitoxin ( mAb ) Anthrax Marketed Product
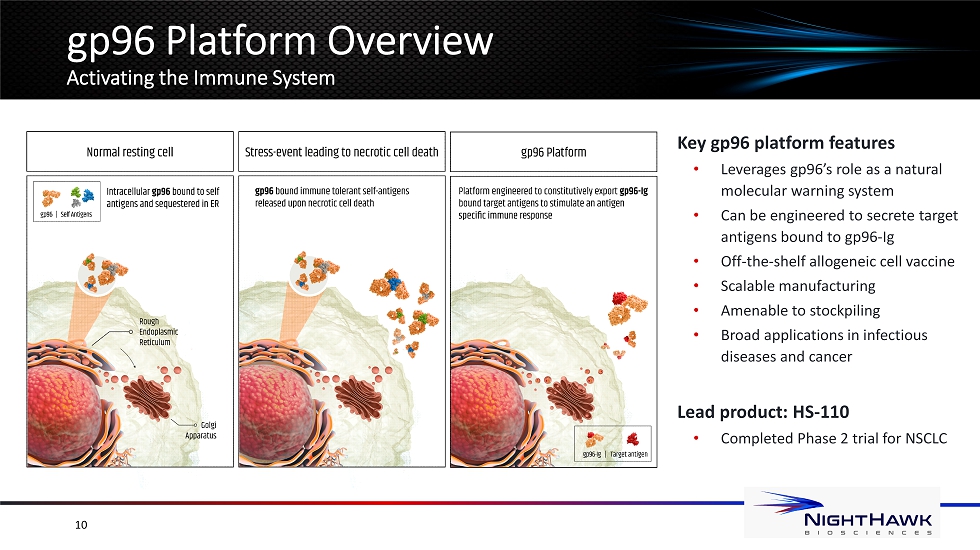
gp96 Platform Overview Activating the Immune System Key gp96 platform features • Leverages gp96’s role as a natural molecular warning system • Can be engineered to secrete target antigens bound to gp96 - Ig • Off - the - shelf allogeneic cell vaccine • Scalable manufacturing • Amenable to stockpiling • Broad applications in infectious diseases and cancer Lead product: HS - 110 • Completed Phase 2 trial for NSCLC 10
HS - 110 ( viagenpumatucel - L) gp96 - Based Cancer Vaccine Targeting Solid Tumors HS - 110 is a first - in - class, “off - the - shelf”, allogeneic cell - based immunotherapy • Engineered to secrete a wide range of cancer - associated antigens bound to the immunostimulatory chaperone gp96 • Designed to stimulate and facilitate uptake of cancer antigens by antigen presenting cells (APCs), which in turn activate a broad, T - cell medicated immune response against a patient’s cancer • Worldwide rights available Enrollment complete for Phase 2 NSCLC evaluating HS - 110 in combination with PD - 1 therapy • Positive interim survival data in previously treated PD - (L)1 naïve and PD - (L)1 progressor NSCLC patients • Plan to discuss registrational pathways with potential partners Combination of HS - 110 and PD - (L)1 therapies may confer additional survival benefit in multiple cancers • Line extension strategy to include additional indications that have been approved for PD - (L)1 therapies 11
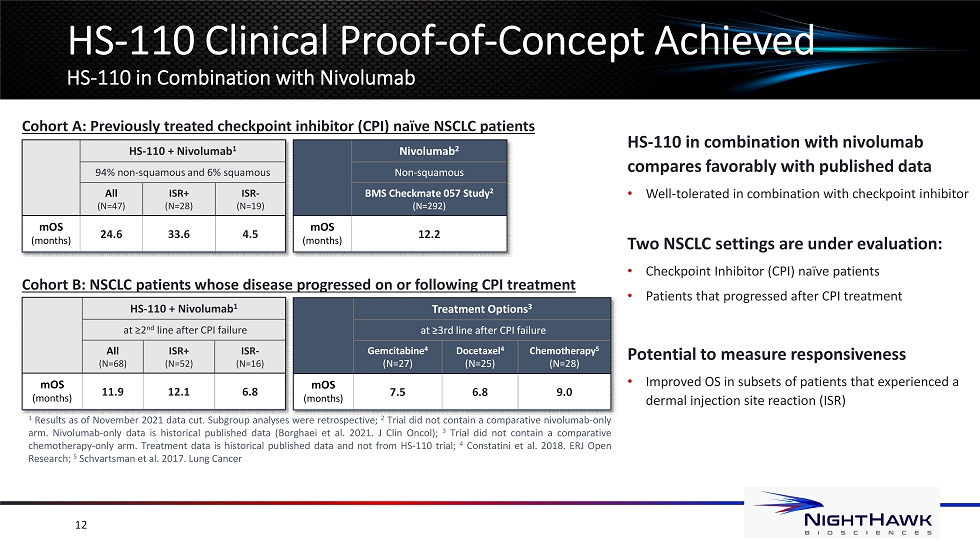
12 HS - 110 Clinical Proof - of - Concept Achieved HS - 110 in Combination with Nivolumab HS - 110 + Nivolumab 1 94% non - squamous and 6% squamous All (N=47) ISR+ (N=28) ISR - (N=19) mOS (months) 24.6 33.6 4.5 Nivolumab 2 Non - squamous BMS Checkmate 057 Study 2 (N=292) mOS (months) 12.2 HS - 110 + Nivolumab 1 at ≥2 nd line after CPI failure All (N=68) ISR+ (N=52) ISR - (N=16) mOS (months) 11.9 12.1 6.8 Treatment Options 3 at ≥3rd line after CPI failure Gemcitabine 4 (N=27) Docetaxel 4 (N=25) Chemotherapy 5 (N=28) mOS (months) 7.5 6.8 9.0 Cohort A: Previously treated checkpoint inhibitor (CPI) naïve NSCLC patients Cohort B: NSCLC patients whose disease progressed on or following CPI treatment 1 Results as of November 2021 data cut . Subgroup analyses were retrospective ; 2 Trial did not contain a comparative nivolumab - only arm . Nivolumab - only data is historical published data ( Borghaei et al . 2021 . J Clin Oncol) ; 3 Trial did not contain a comparative chemotherapy - only arm . Treatment data is historical published data and not from HS - 110 trial ; 4 C onstatini et al . 2018 . ERJ Open Research ; 5 Schvartsman et al . 2017 . Lung Cancer HS - 110 in combination with nivolumab compares favorably with published data • Well - tolerated in combination with checkpoint inhibitor Two NSCLC settings are under evaluation: • Checkpoint Inhibitor (CPI) naïve patients • Patients that progressed after CPI treatment Potential to measure responsiveness • Improved OS in subsets of patients that experienced a dermal injection site reaction (ISR)
PTX - 35 Overview Potential First - in - Class TNF Receptor Superfamily Member 25 (TNFRSF25) Agonist Antibody PTX - 35 targets TNFRSF25 to shift the balance between inflammation and immunosuppression • Context driven T cell responses depending on activation signals and disease setting • Dynamic immunomodulatory properties of TNFRSF25 make it a compelling therapeutic target for context - dependent regulation of T cell responses and immune stability • Favorable safety profile demonstrated in non - human primates Phase 1 trial evaluating safety of PTX - 35 treatment for solid tumors • Anti - tumor activity demonstrated in preclinical colon, lung and breast cancer models • Preclinical data demonstrate anti - tumor activity, expansion of antigen - specific CD8 + T cells and decreased Treg suppression in the presence of tumor antigen (AACR 2021) • Awarded a $15.2M CPRIT grant to fund Phase 1 clinical development Worldwide rights licensed by NightHawk Biosciences 13
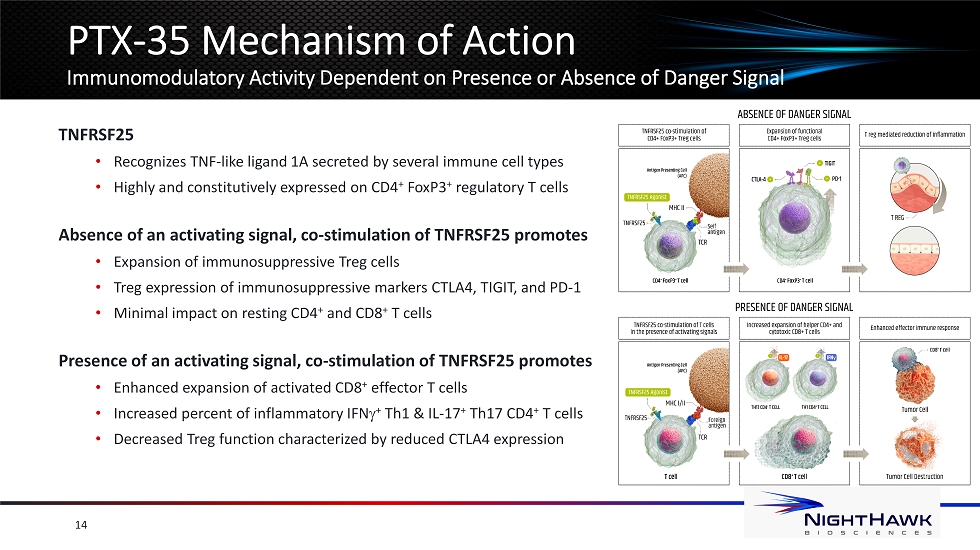
PTX - 35 Mechanism of Action Immunomodulatory Activity Dependent on Presence or Absence of Danger Signal 14 TNFRSF25 • Recognizes TNF - like ligand 1A secreted by several immune cell types • Highly and constitutively expressed on CD4 + FoxP3 + regulatory T cells Absence of an activating signal, co - stimulation of TNFRSF25 promotes • Expansion of immunosuppressive Treg cells • Treg expression of immunosuppressive markers CTLA4, TIGIT, and PD - 1 • Minimal impact on resting CD4 + and CD8 + T cells Presence of an activating signal, co - stimulation of TNFRSF25 promotes • Enhanced expansion of activated CD8 + effector T cells • Increased percent of inflammatory IFN g + Th1 & IL - 17 + Th17 CD4 + T cells • Decreased Treg function characterized by reduced CTLA4 expression

Anthim® Overview Best - in - Class Antitoxin for the Treatment of Anthrax Anthim treats & protects against inhalational anthrax disease • Monoclonal antibody that binds protective antigen (PA83) released by bacillus anthracis • Neutralizes anthrax toxin • In combination with antibiotics or prophylaxis when alternative therapies are not available • For complete prescribing information including limitations of use and box safety warning associated with HYPERSENSITIVITY and ANAPHYLAXIS, see https://anthim.com/ US FDA approval in 2016; CA, EU and UK approval as of 2020 • Only anthrax antitoxin to have received international licensure Anthim is supplied to the US Strategic National Stockpile • As part of ASPR’s objective to diversify supply and acquire products with a longer shelf - life, completed and shipped 2 orders totaling $70M in 2016 and 2018 • Completed first phase of contract for $50 million; HHS options to procure up to $31 million of Anthim by the first half of 2023 15
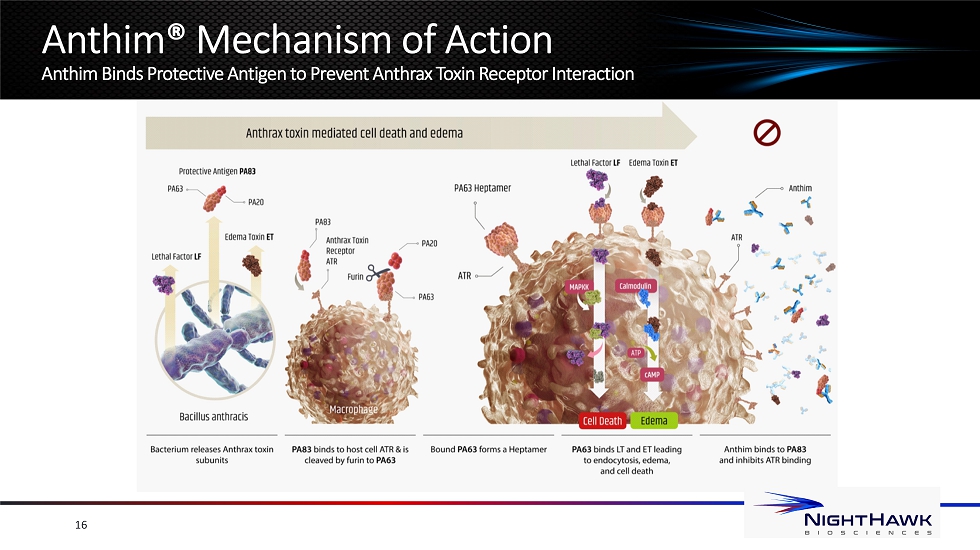
16 Anthim® Mechanism of Action Anthim Binds Protective Antigen to Prevent Anthrax Toxin Receptor Interaction

RapidVax ® Platform Overview A Customizable Approach to Countering Emerging Biological Threats Novel “plug - and - play” vaccine platform • Premanufactured gp96 - Ig/OX40L - Ig stockpile - amenable cells - potential to reduce time from identification to immunization • Target antigen sequences transfected into premanufactured RapidVax to rapidly create a pathogen - specific vaccine • Engineered for potential long - term protection via the stimulation of antibody production and immunological memory • Leverages favorable clinical trial safety profile previously observed for the gp96 platform 17
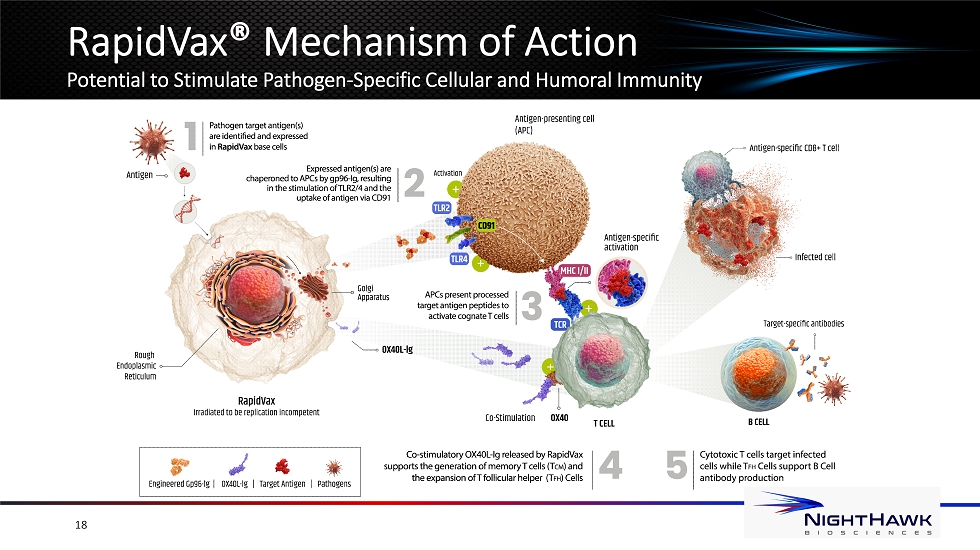
RapidVax ® Mechanism of Action Potential to Stimulate Pathogen - Specific Cellular and Humoral Immunity 18

Biothreat Advisory Board Bipartisan Board Providing Counsel on N IGHT H AWK’s Medical Countermeasure Initiatives 19 Andrew Weber Jack Kingston Dr. Gregory Koblentz Mark Pryor Former Deputy Asst. Sec. of Defense for Countering Weapons of Mass Destruction Former Asst. Sec. of Defense for Nuclear, Chemical & Biological Defense Programs Former US Representative, Secretariat of the Alliance for Biosecurity (current) Professor of Biodefense at George Mason University, Expert on Chemical and Biological Weapons Former US Senator, AR David Lasseter Greg Burel Gen. Richard Myers (Chairman) Former Director of the Strategic National Stockpile Former Chairman of the Joint Chiefs of Staff
N IGHT H AWK Highlights Marketed product and robust pipeline of immunomodulators spanning oncology, inflammation, and infectious disease • FDA approved Anthim ® ( obiltoxaximab ), best - in - class antitoxin for inhalation anthrax • Multiple biologics advanced from bench to clinic: HS - 110 and HS - 130 (allogenic cell therapies), PTX - 35 (antibody - based therapy) • RapidVax ® in development as a novel vaccine platform designed to a ccelerate time to clinic to combat emerging biological threats • Biothreat Advisory Board provides guidance on medical countermeasure initiatives End - to - end ecosystem designed to accelerate the development of discoveries to the delivery of novel therapeutics • Skunkworx Bio unique p roprietary biologics discovery platform and computational approach to drive intelligen t target identification • Heat Biologics and Pelican Therapeutics preclinical and clinical expertise support efficient trial design and execution • Elusys Therapeutics sophisticated knowledge and hands - on experience in medical countermeasures biologics and government funding • Scorpion Biological Services cGMP biomanufacturing and analytical services – 500,000+ sq ft commercial facility expansion in pro gress

21 N IGHT H AWK B IOSCIENCES PLEASE NOTE: Heat Biologics, Inc anticipates transitioning to NightHawk Biosciences, Inc effective May 3, 2022.