PRESENTATION
Published on May 28, 2014

Corporate Presentation
May 2014

Forward Looking Statements
This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking
statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,”
“expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations
thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of
places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current
expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem
cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of
and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner
our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding
clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will
be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing,
the industry in which we operate and the trends that may affect the industry or us.
statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,”
“expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations
thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of
places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current
expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem
cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of
and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner
our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding
clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will
be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing,
the industry in which we operate and the trends that may affect the industry or us.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and
healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future
or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-
looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future
performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we
operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the
factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2013 and our
quarterly report on Form 10-Q for the quarter ended March 31, 2014 (collectively, our “SEC Filings”). In addition, even if our results of
operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-
looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-
looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to
update such statements to reflect events or circumstances after the date of this presentation, except as required by law.
healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future
or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-
looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future
performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we
operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the
factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2013 and our
quarterly report on Form 10-Q for the quarter ended March 31, 2014 (collectively, our “SEC Filings”). In addition, even if our results of
operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-
looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-
looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to
update such statements to reflect events or circumstances after the date of this presentation, except as required by law.
You should read carefully our “Special Cautionary Notice Regarding Forward-Looking Statements” and the factors described in the
“Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business.
“Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business.
2

Investor Highlights
4
Ø Transformative, differentiated immunotherapy platform generating
diverse pipeline with strong patent estate
diverse pipeline with strong patent estate
Ø Promising monotherapy clinical data in lung cancer with impressive
survival results
survival results
Ø 2 clinical development programs advancing into Phase 2 studies in
2014 expected to generate key near-term catalysts
2014 expected to generate key near-term catalysts
Ø Robust business development initiative with potential for multiple
regional and global partnering opportunities
regional and global partnering opportunities
Ø Experienced team with extensive oncology operational, scientific,
clinical and business development expertise
clinical and business development expertise

Management Team with Proven Track Record
5
Jeff Wolf
Founder and CEO
• Founder and managing director of Seed-One Ventures
• Co-founder and director, Avigen
• Co-founder and Chairman, TyRx Pharma
• Founder and CEO, EluSys Therapeutics
Matt Czajkowski
Chief Financial Officer
• Fifteen years experience as Chief Financial Officer for a
variety of early stage and public companies: Pozen, Inc., AAI
Pharma, Incventure funded private companies
variety of early stage and public companies: Pozen, Inc., AAI
Pharma, Incventure funded private companies
• Chief Executive Officer of NextRay, Inc.
• Investment Banker, Goldman Sachs & Co.’s Asia Pacific
Mergers and Acquisition Group in Tokyo, Japan.
Mergers and Acquisition Group in Tokyo, Japan.
Anil K. Goyal, Ph.D.,
VP, Business Development
• 20 years of experience at private and public biotechnology
companies covering all aspects of licensing, deal making,
companies covering all aspects of licensing, deal making,
and strategic alliances
• Management and BD roles with Serenex, Inc., Millennium
Pharmaceuticals, Genome Therapeutics Corporation,
Qualiber, and Ascletis Pharmaceuticals.
Pharmaceuticals, Genome Therapeutics Corporation,
Qualiber, and Ascletis Pharmaceuticals.
Melissa Price, Ph.D.
VP, Clinical and Reg. Affairs
• Led numerous oncology programs in both the biotech arena
and the CRO space
• Leadership roles at INC Research, Novaquest, (a Quintiles
Company and Attenuon
Company and Attenuon
• Published in numerous scientific journals
Taylor Schreiber, M.D., Ph.D.
VP, Research and Development
• Co-inventor of significant elements of ImPACT technology
platform
platform
• Co-inventor of TNFRSF25 agonist technology
• Author of numerous immunology and heat shock protein-based
cancer immunotherapy publications
cancer immunotherapy publications
• Post-doctoral fellowship with Eckhard R. Podack, M.D., Ph.D., at
University of Miami
University of Miami

World Renowned Advisory Boards
6
Scientific Advisory Board
Clinical Advisory Board
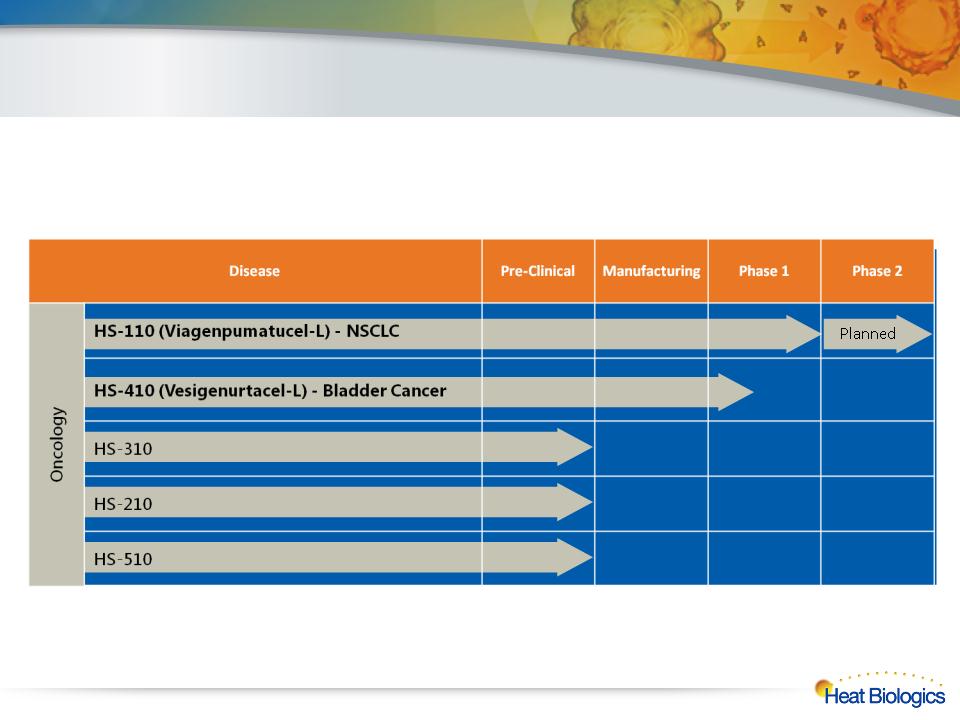
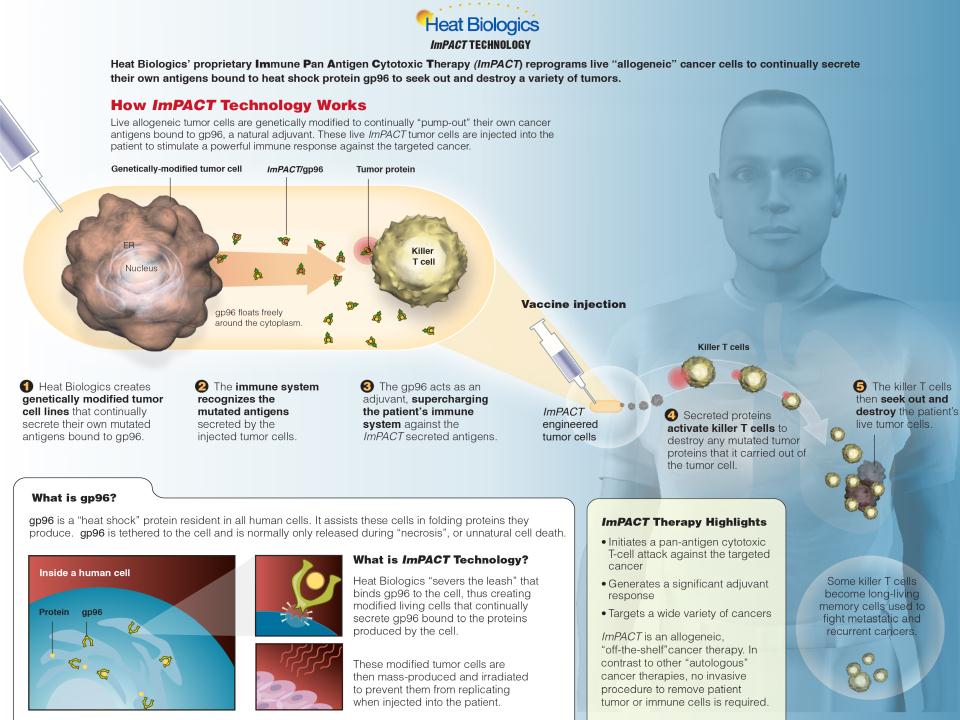
Diverse Pipeline with Multiple Registration Opportunities
7
From drug discovery to late-stage clinical development
IP Estate with Broad and Early Filings
Broad Issued Patents on ImPACT Platform & Pipeline
ImPACT Platform Technology
l US and foreign patents issued for ImPACT technology for the treatment of cancer
and viral disease
l Additional patents on proprietary cell lines and clinical data
Worldwide Filings
l Over 50 patent applications across 5 patent families
l Enforceable patents issued in 15 countries and counting
8
Heat’s ImPACT Therapy
Living Drug Factories
Antigen and adjuvant delivery in a single package
9

Introducing gp96 — Immune System’s “Swiss Army Knife”*
“Molecular Warning System”
* Schild, H. & Rammensee, H. Gp-96 - The Immune System’s Swiss Army Knife. Nature Immunology 2, 100
-101 (2000)
-101 (2000)
gp96
antigen
leash
l Natural biological process to deliver proteins (antigens)
+ gp96 adjuvant to our immune system
+ gp96 adjuvant to our immune system
l Gp96 “chaperones” newly-created proteins to the cell
membrane where they are released and embedded
membrane where they are released and embedded
l Activates a cytotoxic T-cell response to the antigen it is
carrying when cells die through necrosis
carrying when cells die through necrosis
– Enables MHC I antigen cross-presentation to CD8+ T-cells
l Gp96 + protein are only naturally released via necrosis
– Exposure of gp96 outside the cell activates an immune
response to the antigen it is carrying
response to the antigen it is carrying
– Enables MHC I antigen cross-presentation specifically to
CD8+ T-cells
CD8+ T-cells
l Among the most powerful adjuvants and the only
adjuvant to show exclusive specificity to CD8+ (“killer”)
T-cells
adjuvant to show exclusive specificity to CD8+ (“killer”)
T-cells
– Provides long-term immunity against the infectious agent
Tethered to our cells with a “KDEL” leash
Endoplasmic Reticulum
Cell Membrane
Antigen
10
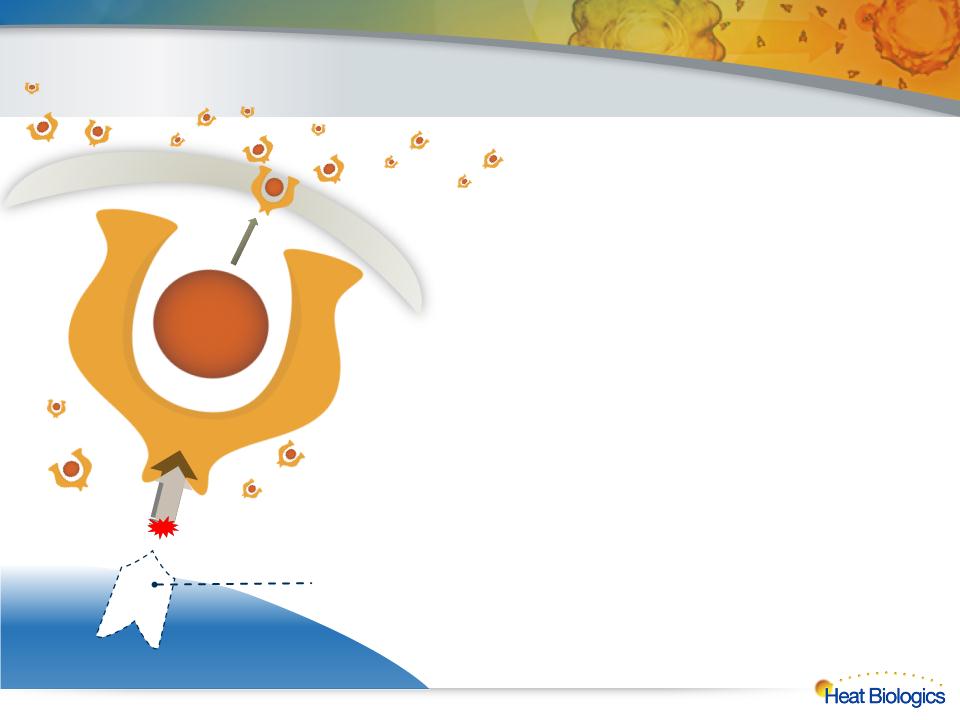
l Genetically modify tumor cells by “severing the
leash” that binds the gp96 to the endoplasmic
reticulum of the cell and replacing it with a
sequence that pumps gp96 out of the cell
leash” that binds the gp96 to the endoplasmic
reticulum of the cell and replacing it with a
sequence that pumps gp96 out of the cell
l Enables living cancer cells to “pump-out” their own
surface antigens along with their gp96 chaperone
surface antigens along with their gp96 chaperone
– Mimics necrotic cell death
l Activates a powerful pan-antigen cytotoxic T-cell
immune response
immune response
l “Off-the-shelf” therapy designed to enable a fully
in vivo attack against a wide variety of cancers
in vivo attack against a wide variety of cancers
Heat Biologics ImPACT technology removes the leash that binds
gp96 to the cell, replacing with a sequence that allows cells to
continually secrete gp96 along with their “chaperoned” antigen
gp96 to the cell, replacing with a sequence that allows cells to
continually secrete gp96 along with their “chaperoned” antigen
Cell Membrane
11
ImPACT Therapy — “Severing the Leash”
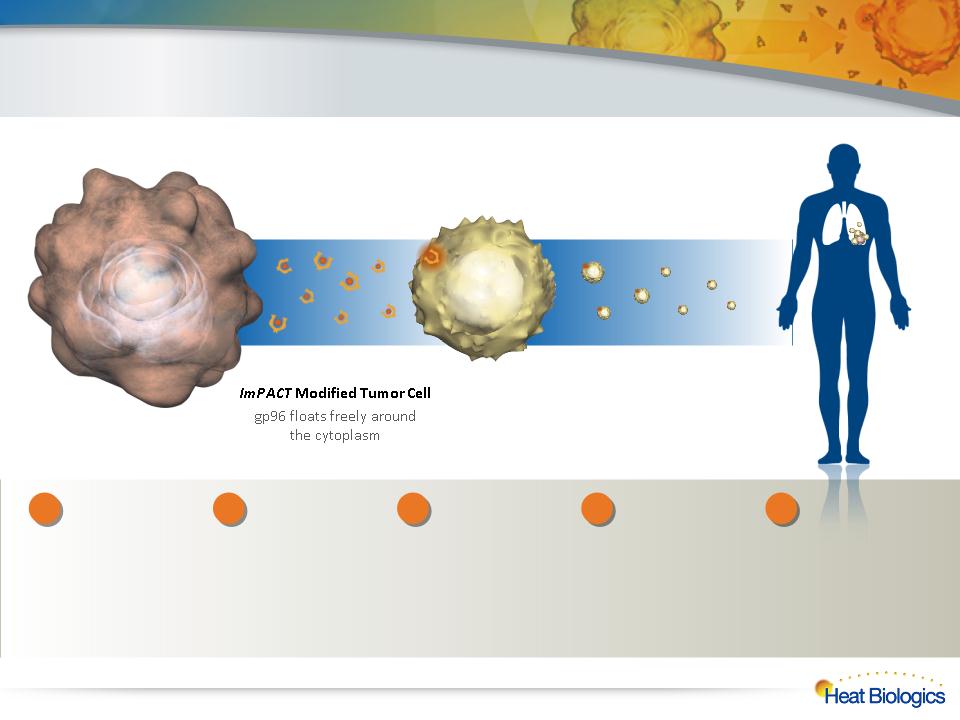
Vaccine Injection
Inject living ImPACT cells
into the patient
into the patient
ImPACT Therapy — Process
Non-functional
Tumor Cell
Tumor Cell
Killer
T-Cell
T-Cell
Heat Biologics
creates
genetically
modified tumor
cell lines to
continually
secrete their own
mutated antigens
bound to gp96.
creates
genetically
modified tumor
cell lines to
continually
secrete their own
mutated antigens
bound to gp96.
2
Scale-up production
of these living tumor
cells as our “drug” to
treat all patients with
a particular cancer.
Irradiate these cells
so they can’t
replicate and vial for
distribution.
of these living tumor
cells as our “drug” to
treat all patients with
a particular cancer.
Irradiate these cells
so they can’t
replicate and vial for
distribution.
3
Inject these living,
genetically-
modified cells into
patients. These
cells continuously
secrete tumor
proteins bound to
gp96.
genetically-
modified cells into
patients. These
cells continuously
secrete tumor
proteins bound to
gp96.
4
Secreted proteins
activate killer T-
cells to seek-out
and destroy the
targeted cancer.
activate killer T-
cells to seek-out
and destroy the
targeted cancer.
5
1
Choose cancer of
interest and
identify a cell line
representative of
that cancer.
interest and
identify a cell line
representative of
that cancer.
12
ImPACT — Highlights
An “Off-the Shelf” Therapy to Generate a Pan-antigen T-cell Immune Attack
Approach
• Allogeneic, “off-the-shelf” treatment
created from a master cell line
created from a master cell line
• No tumor cells, blood or anything else
extracted from the patient
extracted from the patient
• Non-invasive
Benefits
• Unlimited drug supply enables
immediate treatment
• Frequent administration with no patient-
specific processing
specific processing
• Less expensive to produce
and administer than autologous therapies
with COGS < 5% of autologous approaches
with fewer logistical hurdles
and administer than autologous therapies
with COGS < 5% of autologous approaches
with fewer logistical hurdles
• Pan-antigen immune attack
• Unleashes an immune attack against a
wide variety of known and unknown
cancer antigens
wide variety of known and unknown
cancer antigens
• Cytotoxic T-cell exclusive immune response
• Antigen + adjuvant in a single complex
• Antigen + adjuvant presented simultaneously
• Activates robust and highly specific immune
response against secreted cancer antigens
response against secreted cancer antigens
• Continuous secretion of gp96-
antigen/adjuvant complex
antigen/adjuvant complex
• Generates more robust and sustained antigen
-specific immune response
-specific immune response
13
Heat’s HS-110 Therapy
• Cells are genetically modified to secrete gp96
and most known (and many unknown) lung
cancer antigens
and most known (and many unknown) lung
cancer antigens
• Pan-antigen cytotoxic T-cell immune response
Treatment
• Powerful immune activation
• Positive safety profile based on preclinical
studies and one clinical study
studies and one clinical study
• The drug is administered in a simple,
once-a-week injection
once-a-week injection
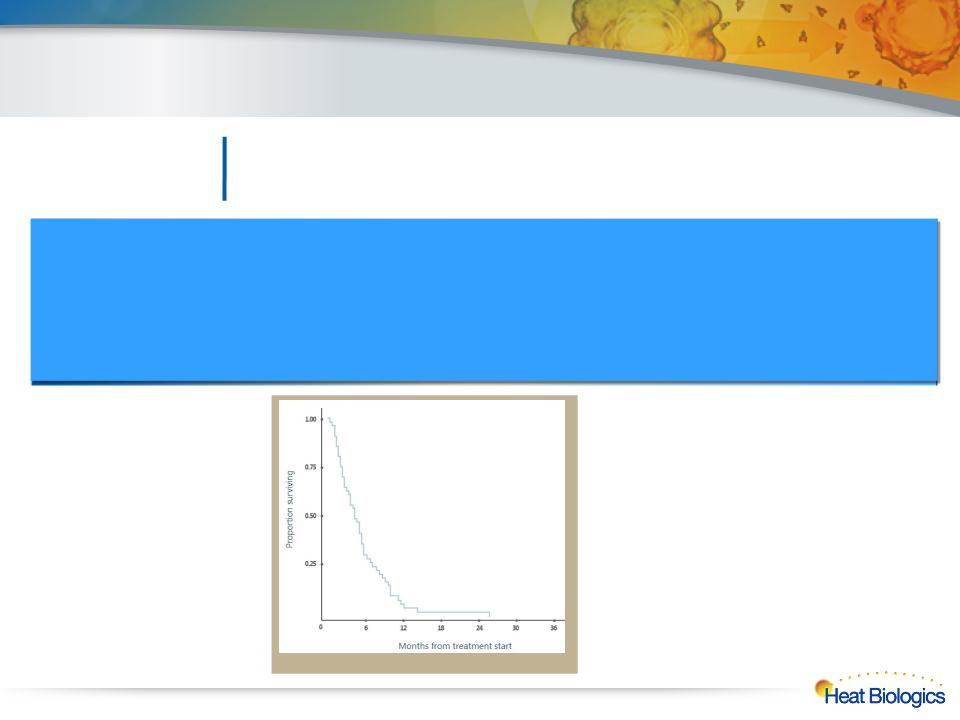
Survival Curve for Late-Stage 3B/4
NSCLC Patients*
NSCLC Patients*
* Massarelli E. Lung Cancer;2003:39 - Meta Analysis
Current Treatment
• Surgical Resection
• Radiation Therapy
• Chemotherapy
– 3-6 cycles
– Each cycle lasts 3-4 weeks
• Targeted Therapies
Survival Prospects
• Median survival ~ 4.5 months*
• 1 year survival ~6%*
Lung Cancer and HS-110 (Viagenpumatucel-L)
Background
“Without any chemotherapy, the average person will live about 4½ months.
With chemotherapy most will live longer and some will live a shorter time. More recent chemotherapy trials have
shown that people live about 3 months longer than if they did not get chemotherapy …
With chemotherapy most will live longer and some will live a shorter time. More recent chemotherapy trials have
shown that people live about 3 months longer than if they did not get chemotherapy …
… Even with chemotherapy, the chance of being alive at one year is about 30-50%;
the chance of dying within this year is 50-70%.”
the chance of dying within this year is 50-70%.”
— American Society for Clinical Oncology (ASCO) Guidelines
Lung Cancer is the Second Most Common Cancer in
US with No Reliable Treatment Options for Late-Stage
Patients
US with No Reliable Treatment Options for Late-Stage
Patients
14
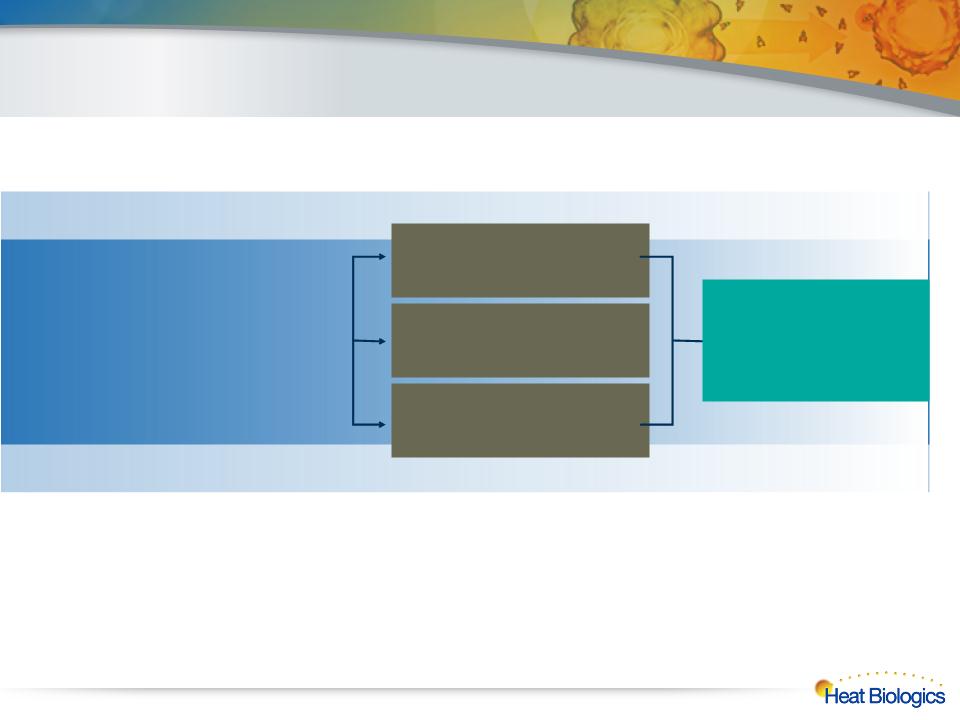
HS-110: Successful Completion of Phase 1 Study
l NIH-funded, open-label, single center investigator-sponsored IND
l 18 patients with late-stage NSCLC
l Enrollment alternating in 3 cohorts after first 8 patients
Stage IIIB/IV NSCLC
Disease progressing at time of Enrollment
Failed multiple lines of prior therapy
Cohort 3
¼ Strength Dose Twice Weekly
¼ Strength Dose Twice Weekly
(10,000,000 Cells)
Cohort 2
½ Strength Dose Weekly
½ Strength Dose Weekly
(20,000,000 Cells)
Cohort 1
1 Dose Every 2 Weeks
1 Dose Every 2 Weeks
(40,000,000 Cells)
Safety
Immune Response,
Tumor Response,
Immune Response,
Tumor Response,
Overall Survival,
Progression Free Survival
Progression Free Survival
15
Phase 1 NSCLC Trial Design
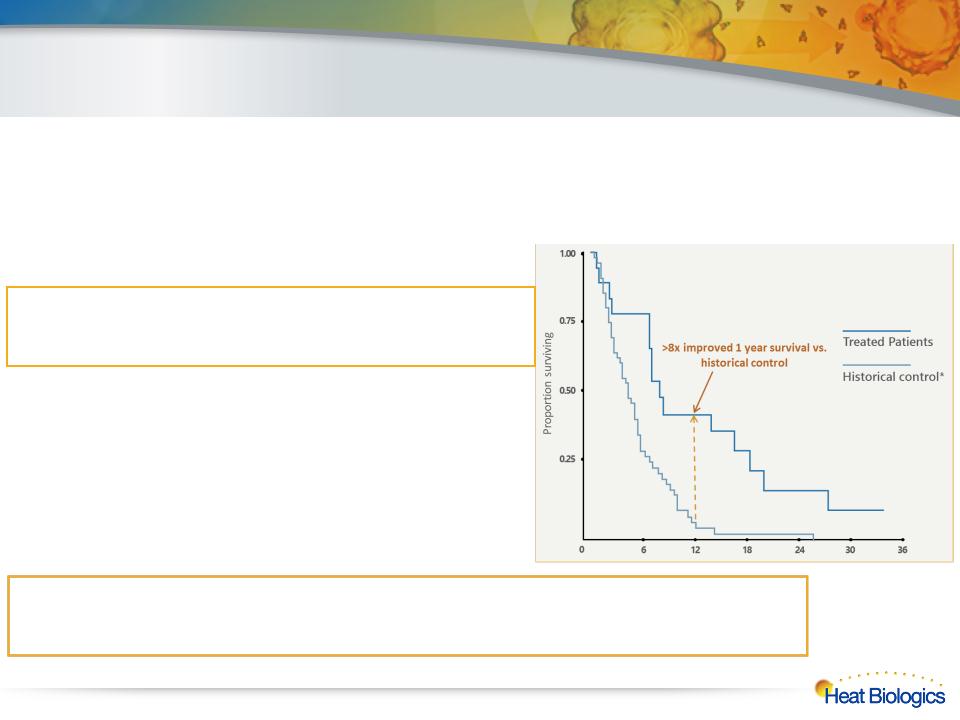
HS-110 NSCLC Phase 1 Clinical Trial Results
16
l Well-tolerated with no overt toxicity
l Single agent clinical activity in late-stage 3b and 4 non-small cell lung cancer
– 7 of 15 surviving patients exhibited stable disease
after single course of therapy
after single course of therapy
l Immune response observed in 73% (11 out of 15)
of patients who completed their first course of
therapy
of patients who completed their first course of
therapy
– Immune response predictive of survival
(HR: 0.021, 95% CI:0.002-0.204)
(HR: 0.021, 95% CI:0.002-0.204)
• The immune responders exhibited a median survival of
16.9 months (95% CI: 7.1-20) while the immune non-
responders exhibited a median survival of 4.5 months
16.9 months (95% CI: 7.1-20) while the immune non-
responders exhibited a median survival of 4.5 months
– Two late-stage patients survive >3 years
• One HS-110 patient alive >3 yrs. and another patient
still alive >4 yrs.
still alive >4 yrs.
l Median 1 year overall survival rate of patients in the study was 44% (95% CI:
21.6-65.1) comparing favorably to a 5.5% rate based on historical control*
21.6-65.1) comparing favorably to a 5.5% rate based on historical control*
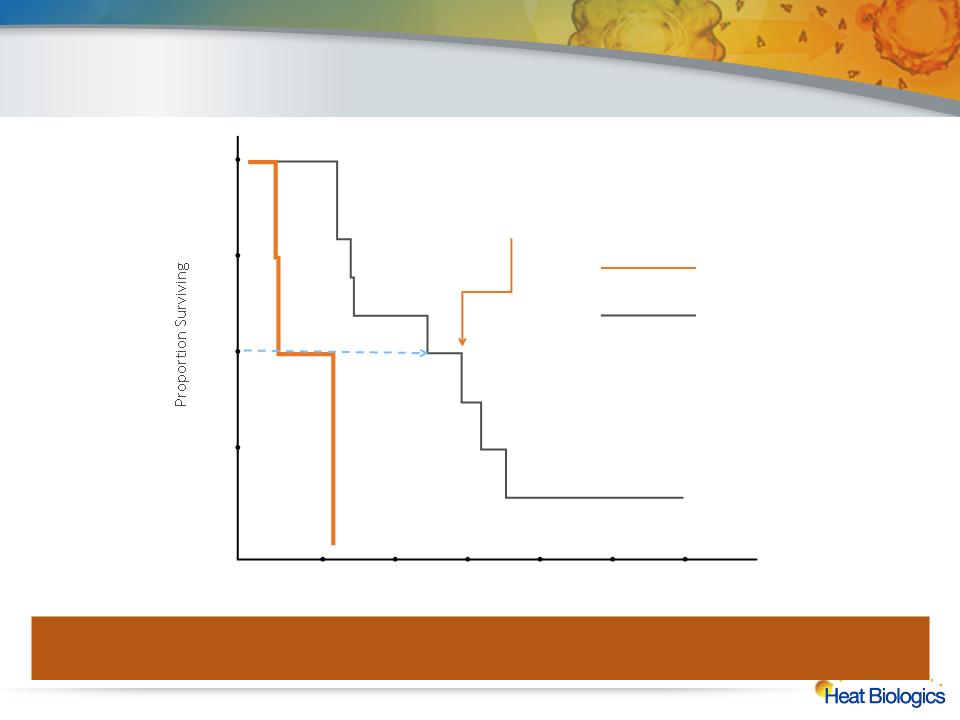
Immune Response Predictive of Survival
In 11 of the 15 patients (73%) completing the first course of therapy with HS-110, there was a twofold or greater increase in CD8 cells
secreting interferon gamma (CD8-CTL IFN-γ) following vaccination. The responders saw a threefold increase in median overall survival
compared to non-responders in the trial, from 4.5 months to 16.9 months
secreting interferon gamma (CD8-CTL IFN-γ) following vaccination. The responders saw a threefold increase in median overall survival
compared to non-responders in the trial, from 4.5 months to 16.9 months
Non-Responders
Responders
>3x Extension of Median OS
for Responders
for Responders
6
12
18
24
30
36
0.25
0.50
1.00
0.75
Months From Treatment Start
0
(N=4)
(N=11)
Hazard Ratio: 0.021
95% CI: 0.002 - 0.204
17

HS-110 Lung Cancer Phase 2 Clinical Program
1H 2014
üFinalize Phase 2 protocol
üSubmit revised protocol to FDA
üAnnounce Phase 2 protocol
Specifically designed in collaboration with leading KOLs to be
complementary in combination with next generation oncology
treatments
complementary in combination with next generation oncology
treatments
2H 2014
§Commence patient enrollment and dosing

Phase 2 Protocol Designed to
Complement Next Generation Oncology Treatments
Complement Next Generation Oncology Treatments
l Address unmet need by offering patients access to investigational agents with
presumably a more favorable safety profile than chemo in a setting where
there is little approved in 3rd-line and the efficacy of these agents is minimal
presumably a more favorable safety profile than chemo in a setting where
there is little approved in 3rd-line and the efficacy of these agents is minimal
l Maximize antigen expression overlap by testing an adenocarcinoma vaccine
to an adenocarcinoma population
to an adenocarcinoma population
l Mimic future combinations with checkpoint inhibitors by utilizing low-dose
cyclophosphamide
cyclophosphamide
l Evaluate the effect of concomitant chemotherapy on the immune response to
HS-110 and of subsequent chemotherapy after HS-110 tumor response
HS-110 and of subsequent chemotherapy after HS-110 tumor response
l Evaluate multiple endpoints due to short time to event in this population
(overall survival, objective response, disease control rate, PFS, immune
response, 6-mo OS, 12-mo OS)
(overall survival, objective response, disease control rate, PFS, immune
response, 6-mo OS, 12-mo OS)
l Capture pre- and post-treatment biopsy tissue when appropriate in order to
correlate antigen expression, TILs and T-cell receptor sequences with
outcomes, potentially leading to proof of concept and precision in patient
selection
correlate antigen expression, TILs and T-cell receptor sequences with
outcomes, potentially leading to proof of concept and precision in patient
selection
19
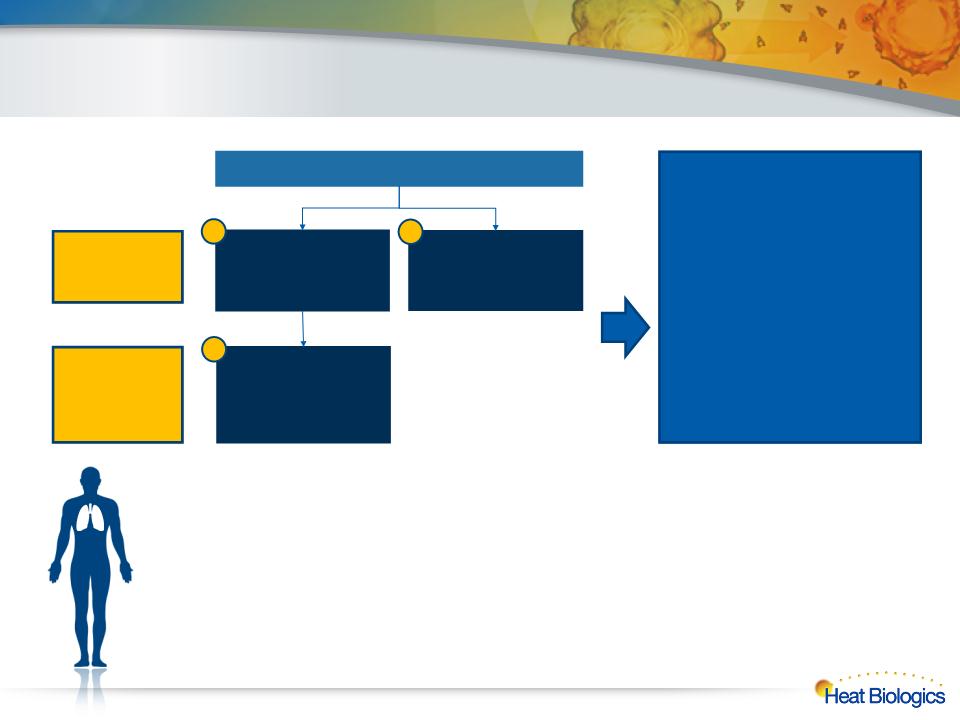
Phase 2 HS-110/CY Combo NSCLC Design
3rd-line vs. physician’s choice, continue vaccine in combo with 3L chemo after 1st progression
3rd-line vs. physician’s choice, continue vaccine in combo with 3L chemo after 1st progression
Randomized
HS-110 + low-dose
CY
CY
Vinorelbine OR
erlotinib OR
gemcitabine OR
paclitaxel
erlotinib OR
gemcitabine OR
paclitaxel
Endpoints
Primary: overall survival
Secondary: immune response
in blood,
in blood,
PFS, DCR, ORR by irRC and
RECIST
RECIST
6-mo OS, 12-mo OS
Pre- and post-treatment biopsy
tissue for exploratory
biomarker analyses
tissue for exploratory
biomarker analyses
Arm 1; N=82
Arm 2; N=41
3rd-line - Stage
A
A
4th-line - Stage
B
B
HS-110 + Vinorelbine
OR erlotinib OR
gemcitabine OR
paclitaxel
OR erlotinib OR
gemcitabine OR
paclitaxel
First Progression
1
2
3
20
Regimen
l Low-dose cyclophosphamide (CY) 50 mg daily for 7 days q2weeks for 12 weeks or until 1st
progression
progression
l HS-110 107 cells weekly for 12 weeks then q3 weeks until 2nd irPD or 12 months
whichever comes first
whichever comes first
Sample Size
l 123 patients randomized 2:1
l 80% power with alpha = 0.1 to detect a 50% reduction in the risk of death with 59 events
in the experimental group and 33 events in the control group
in the experimental group and 33 events in the control group
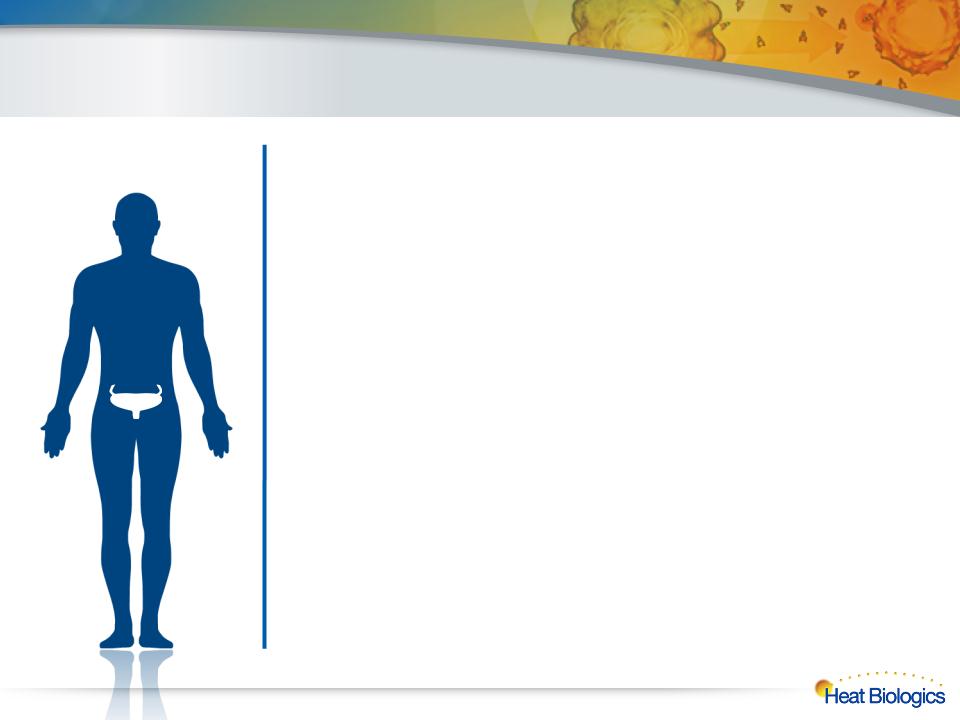
Bladder Cancer and HS-410
Background
l Currently-available treatments have high
failure rate and are poorly tolerated
failure rate and are poorly tolerated
l Among highest lifetime treatment cost per
patient of any cancer due to a high recurrence
rate
patient of any cancer due to a high recurrence
rate
l Opportunity to treat patients with minimal
residual disease
residual disease
l No new drug for this patient population in
>25 years
>25 years
In 2012 Alone, There Were 73,000 New Cases
of Bladder Cancer Reported and 15,000 Deaths
of Bladder Cancer Reported and 15,000 Deaths
21
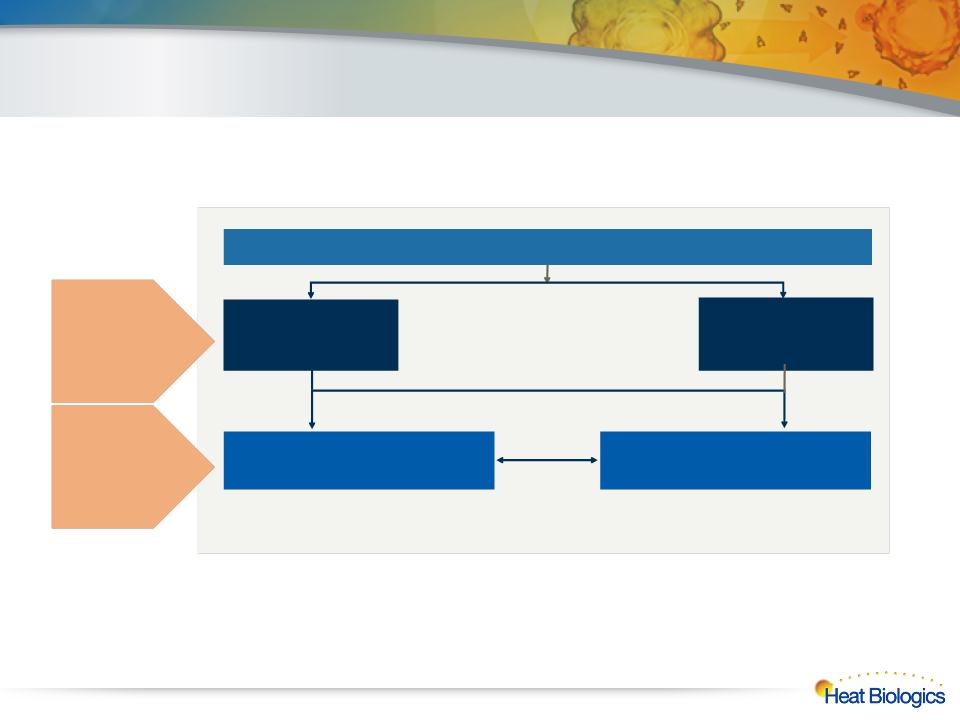
HS-410: Bladder Cancer Phase 1/2
l Population includes non-muscle invasive disease, treated with surgery
followed by 3-6 weeks of BCG therapy
followed by 3-6 weeks of BCG therapy
Placebo
N=25
HS-410
Optimal dose N=50
Optimal dose N=50
Phase 1/2 - 2 Stage Adaptive Design
Proceed with Optimal Dose
HS-410
Cohort 1
Low Dose N=9
1,000,000 cells
HS-410
Cohort 2
High Dose N=9
10,000,000 cells
93 patient, fully-randomized, placebo-controlled trial
Weekly injections for 12 weeks followed by 3 monthly injections
Trial objectives include safety and
immune response
immune response
Trial objectives include safety, immune
response and time to recurrence
response and time to recurrence
22
Stage 1
N = 18
Dose Finding
Stage 2
N = 75
Proof of Concept

Key Study Timelines and Milestones
|
Milestone
|
Target Completion
|
|
First patient enrolled and dosed
|
ü Q1 2014
|
|
Cohort 1 enrollment complete
|
Q3 2014
|
|
Cohort 2 enrollment initiates
|
Q4 2014
|
|
Cohort 1 safety and antigen profiles
|
Q1 2015
|
|
Cohort 2 enrollment complete
|
Q1 2015
|
|
Cohort 2 safety and antigen profiles
|
Q1 2015
|
|
Phase 2 enrollment initiates
|
Q1 2015
|
23
Phase 1/2 Clinical Trial for Bladder Cancer
Business Development Strategy
1. ImPACT Platform Partnerships
• Strategy to Partner by indication(s)
• Use platform for new product discovery
• Product development by partner
2. Clinical Programs (HS-110, HS-410)
• Partner at/after Phase 2 data
• Partner with regional or global rights
• Retain US commercialization rights
• Explore co-development partnerships with anti-PD1 & anti-PDL1 products
24
|
Companies
|
Date
|
Financials
|
|
AZ-Immunocore
|
Jan ‘14
|
$20M+300M/
program |
|
Novartis-UPenn
|
Aug ‘12
|
$20M+MS
|
|
Sanofi-CureVac
|
Nov ‘11
|
$33M+200M
|
Selected Immunotherapy Deals

Financial Snapshot
25
|
Ticker
|
NASDAQ: HTBX
|
|
Shares Outstanding
|
6,452,341
|
|
Share Price*
|
$4.77
|
|
Market Capitalization*
|
~$30M
|
|
Cash as of 3/31/14
|
$19.4M
|
|
Enterprise Value
|
~$10M
|
Financial strength to execute on our business plan
*as of market close on May 22, 2014
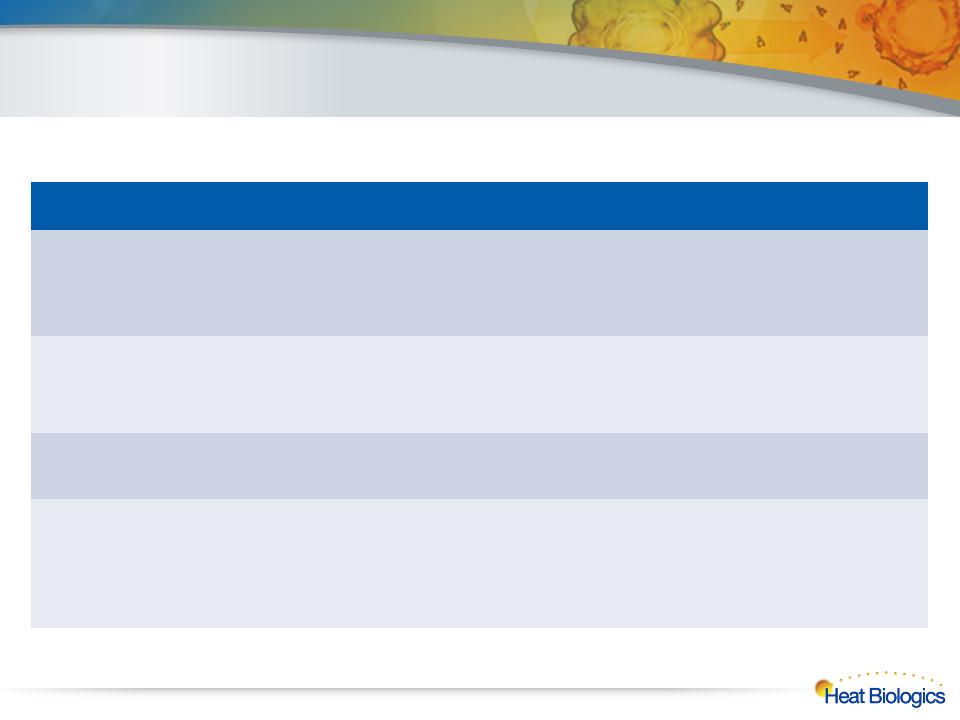
Multiple Near-Term Milestones
Expected to Build Momentum
Expected to Build Momentum
26
|
Program
|
1H 2014
|
2H 2014
|
|
HS-110
Lung Cancer
Program |
ü Submit revised protocol to FDA
|
qInitiate Phase 2 enrollment
|
|
HS-410
Bladder Cancer
Program |
ü Commence patient dosing
|
qComplete Cohort 1 enrollment
qCohort 1 immune response data
qInitiate Cohort 2 enrollment
|
|
3rd Product
|
qGenerate multiple product
candidates |
qAnnounce 3rd product program
|
|
Corporate
|
q Seek development and commercialization partners
q Continued grant filings and notifications
q Additional research developments
q Various clinical publications
|
|

Summary
Clinical Stage Platform Technology Generating Promising Human Data
Clinical Stage Platform Technology Generating Promising Human Data
Data to Date Demonstrate:
• Positive safety profile
• Powerful, disease-specific immune activation
• Immune activation corresponds with increased overall survival
Encouraging
Clinical Data
Strong Clinical Pipeline
• Phase 2 NSCLC clinical trial and Phase 1/2 bladder cancer trial with
additional IND submissions planned in other cancer indications
additional IND submissions planned in other cancer indications
• Multiple near-term enrollment and data readouts
• Potential for business development licensing activity
Value Creating
Milestones
Milestones
Transformational
Technology Platform
Technology Platform
Unleashes the immune system against a wide range of
cancers
cancers
• Over a decade of published research and recent clinical data
27
