POWER POINT PRESENTATION
Published on September 8, 2014
EXHIBIT 99.1

Corporate Presentation September 2014

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2013 and our quarterly report on Form 10-Q for the quarter ended March 31, 2014 and June 30, 2014 (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully our “Special Cautionary Notice Regarding Forward-Looking Statements” and the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. *
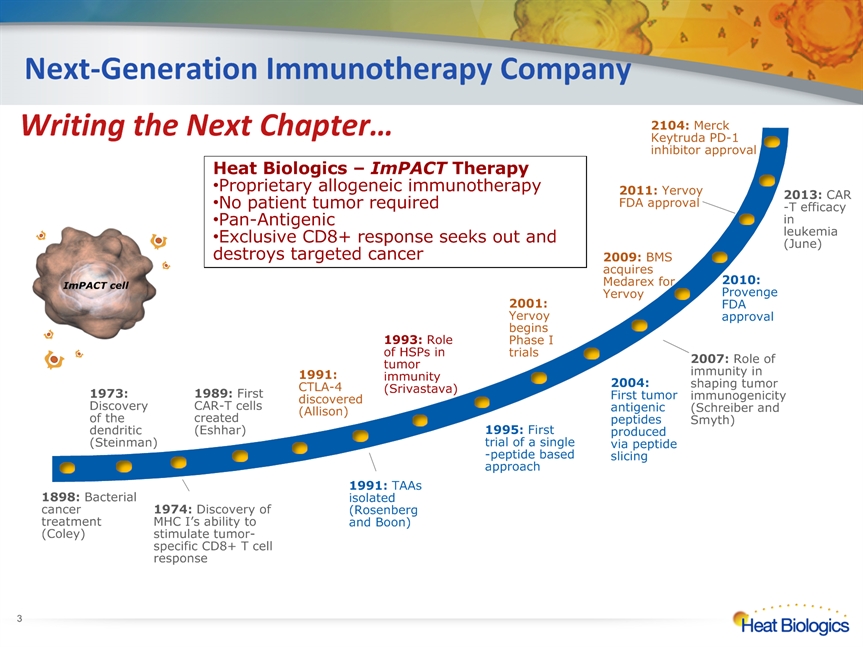
Next-Generation Immunotherapy Company * 1974: Discovery of MHC I’s ability to stimulate tumor-specific CD8+ T cell response Heat Biologics – ImPACT Therapy Proprietary allogeneic immunotherapy No patient tumor requiredPan-AntigenicExclusive CD8+ response seeks out and destroys targeted cancer ImPACT cell 2013: CAR-T efficacy in leukemia (June) Writing the Next Chapter… 2104: Merck Keytruda PD-1 inhibitor approval
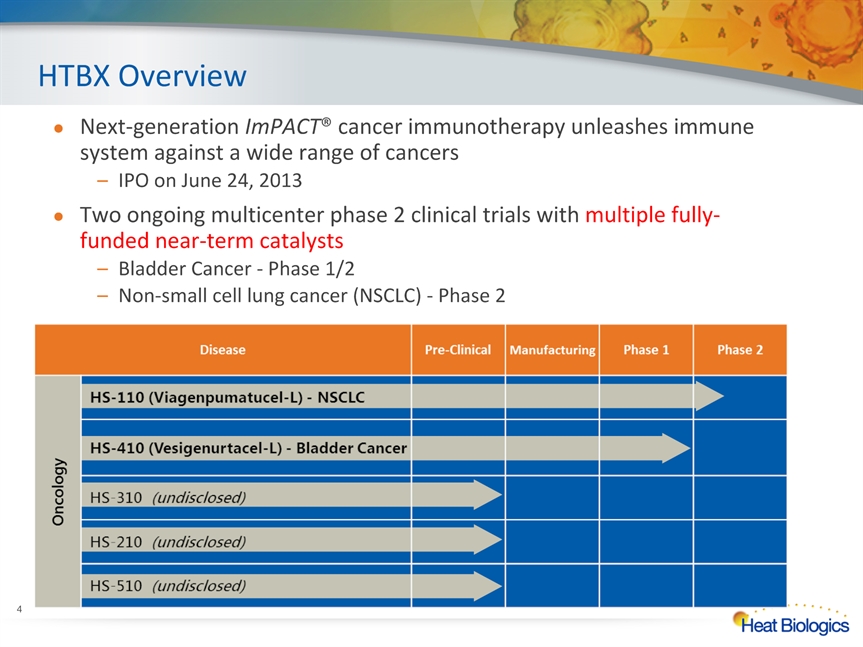
HTBX Overview Next-generation ImPACT® cancer immunotherapy unleashes immune system against a wide range of cancersIPO on June 24, 2013Two ongoing multicenter phase 2 clinical trials with multiple fully-funded near-term catalystsBladder Cancer - Phase 1/2 Non-small cell lung cancer (NSCLC) - Phase 2 *

World-Renowned Advisory Boards * Scientific Advisory Board Clinical Advisory Board
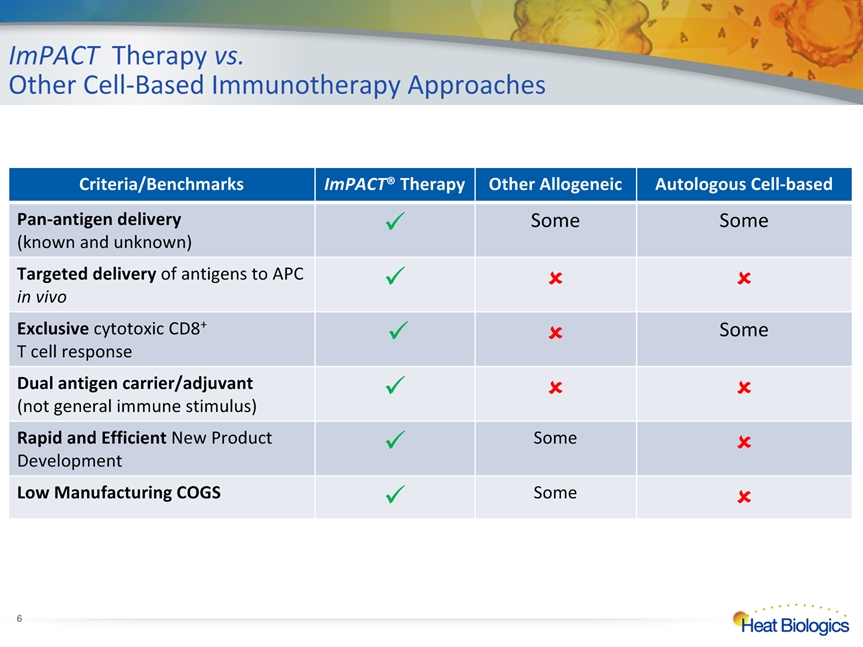
ImPACT Therapy vs. Other Cell-Based Immunotherapy Approaches Criteria/Benchmarks ImPACT® Therapy Other Allogeneic Autologous Cell-based Pan-antigen delivery (known and unknown) Some Some Targeted delivery of antigens to APC in vivo Exclusive cytotoxic CD8+ T cell response Some Dual antigen carrier/adjuvant(not general immune stimulus) Rapid and Efficient New Product Development Some Low Manufacturing COGS Some *
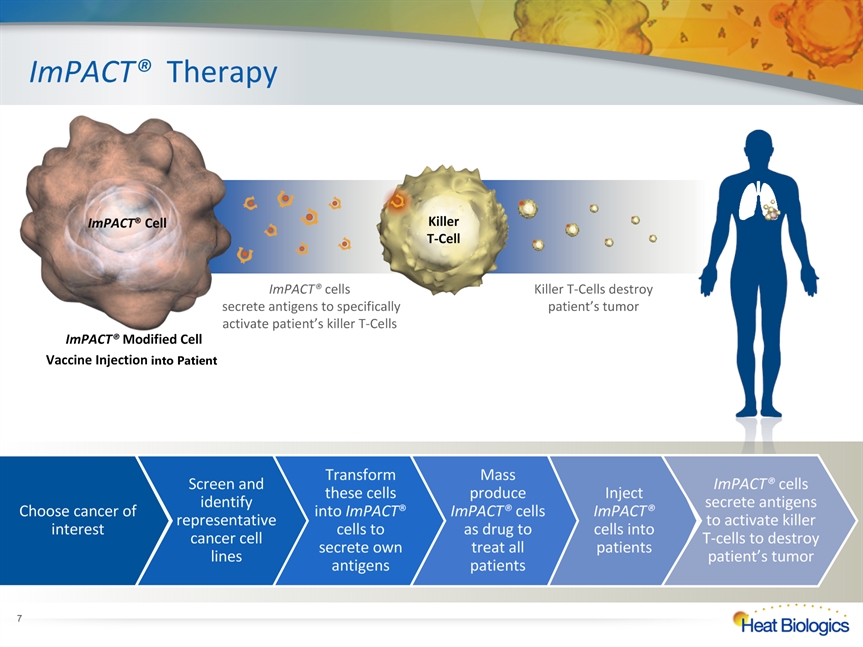
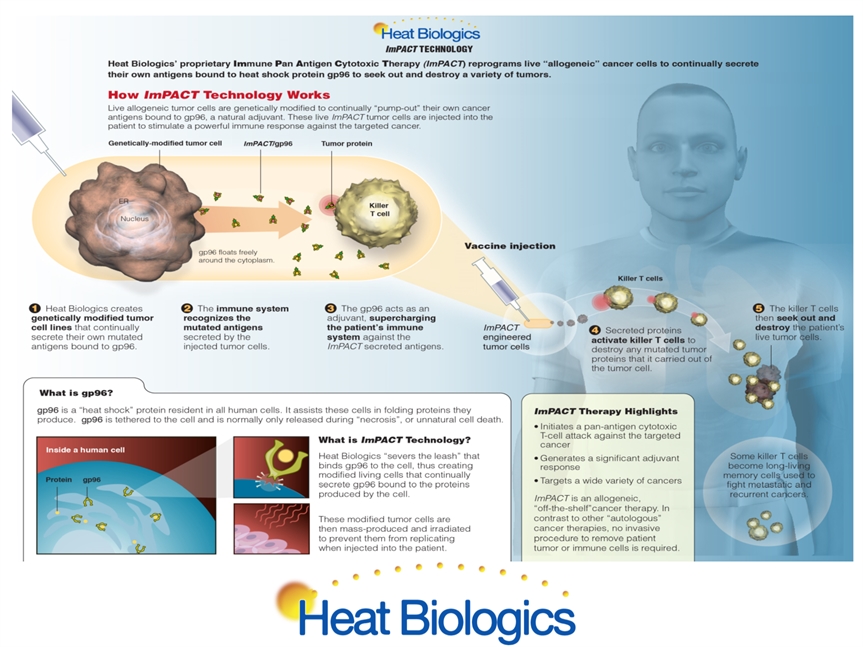
ImPACT® Modified Cell Vaccine Injection into Patient ImPACT® Therapy ImPACT® Cell Killer T-Cell * Choose cancer of interest Screen and identify representative cancer cell lines Transform these cells into ImPACT® cells to secrete own antigens Mass produce ImPACT® cells as drug to treat all patients Inject ImPACT® cells into patients ImPACT® cells secrete antigens to activate killer T-cells to destroy patient’s tumor ImPACT® cells secrete antigens to specifically activate patient’s killer T-Cells Killer T-Cells destroy patient’s tumor
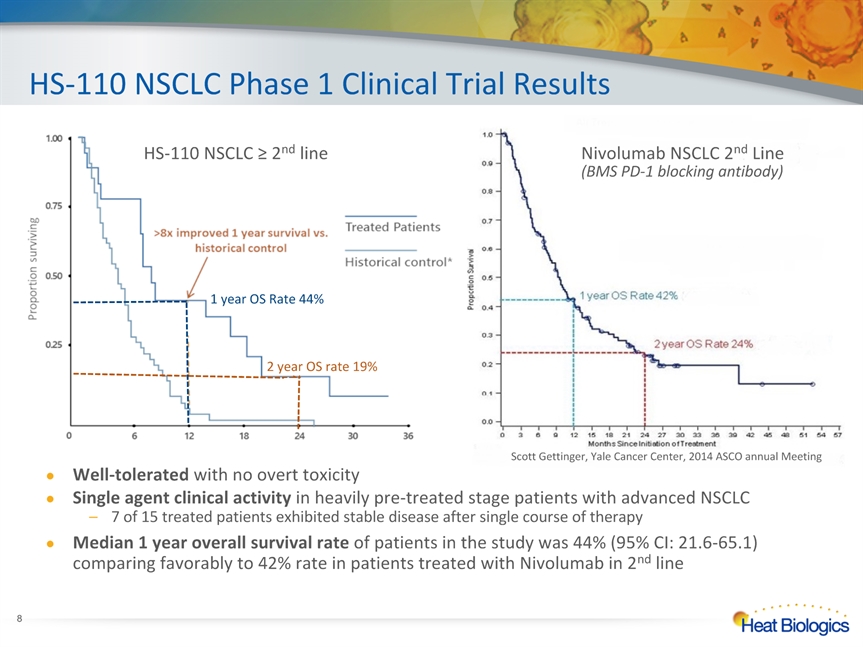
HS-110 NSCLC Phase 1 Clinical Trial Results * Scott Gettinger, Yale Cancer Center, 2014 ASCO annual Meeting Nivolumab NSCLC 2nd Line(BMS PD-1 blocking antibody) HS-110 NSCLC ≥ 2nd line 1 year OS Rate 44% 2 year OS rate 19% Well-tolerated with no overt toxicity Single agent clinical activity in heavily pre-treated stage patients with advanced NSCLC7 of 15 treated patients exhibited stable disease after single course of therapy Median 1 year overall survival rate of patients in the study was 44% (95% CI: 21.6-65.1) comparing favorably to 42% rate in patients treated with Nivolumab in 2nd line
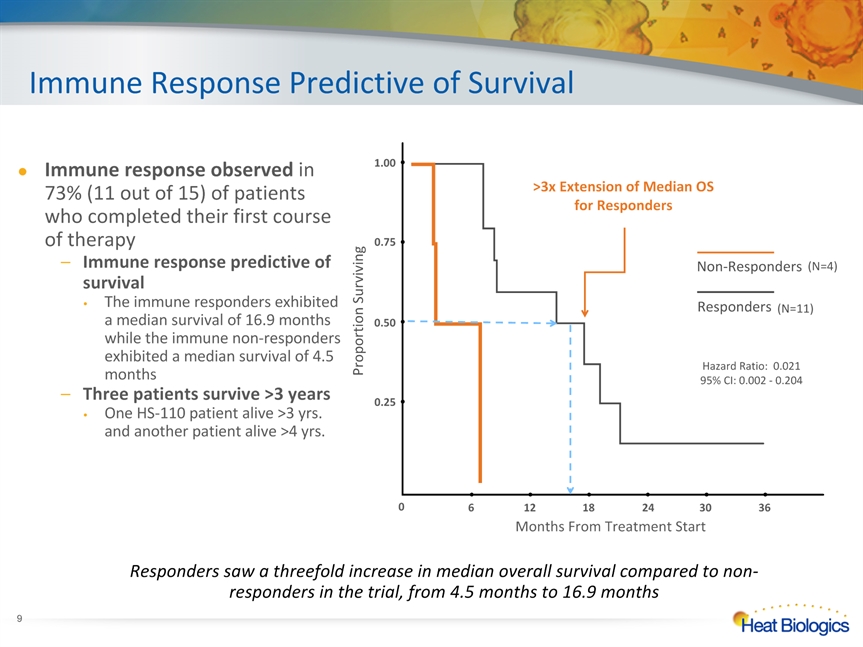
Immune Response Predictive of Survival Responders saw a threefold increase in median overall survival compared to non-responders in the trial, from 4.5 months to 16.9 months Non-Responders Responders >3x Extension of Median OS for Responders 6 12 18 24 30 36 0.25 0.50 1.00 0.75 Proportion Surviving Months From Treatment Start 0 (N=4) (N=11) Hazard Ratio: 0.02195% CI: 0.002 - 0.204 * Immune response observed in 73% (11 out of 15) of patients who completed their first course of therapyImmune response predictive of survival The immune responders exhibited a median survival of 16.9 months while the immune non-responders exhibited a median survival of 4.5 monthsThree patients survive >3 yearsOne HS-110 patient alive >3 yrs. and another patient alive >4 yrs.
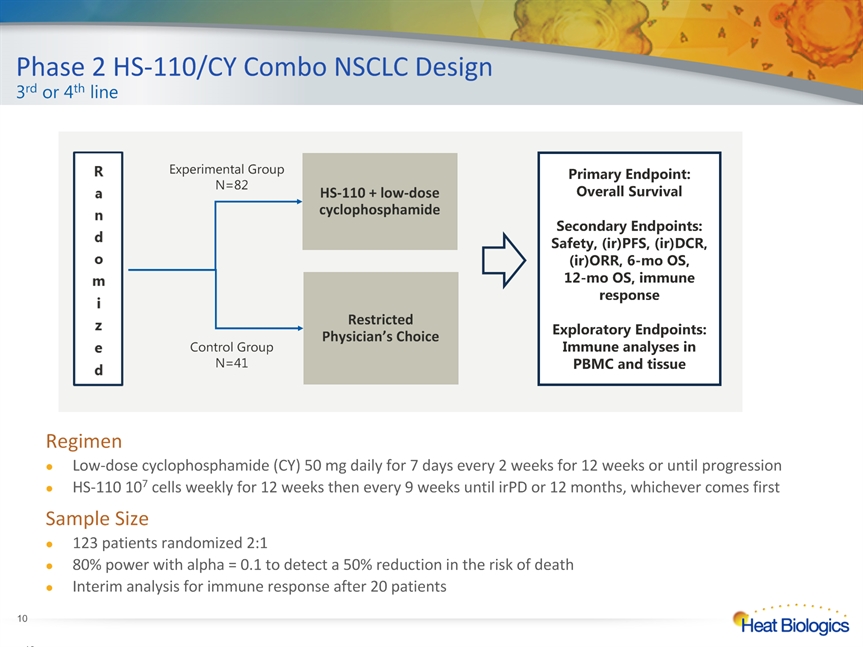
Phase 2 HS-110/CY Combo NSCLC Design3rd or 4th line * * RegimenLow-dose cyclophosphamide (CY) 50 mg daily for 7 days every 2 weeks for 12 weeks or until progressionHS-110 107 cells weekly for 12 weeks then every 9 weeks until irPD or 12 months, whichever comes firstSample Size123 patients randomized 2:1 80% power with alpha = 0.1 to detect a 50% reduction in the risk of death Interim analysis for immune response after 20 patients
Bladder Cancer and HS-410 Background Currently-available treatments have high failure rate and are poorly toleratedAmong highest lifetime treatment cost per patient of any cancer due to a high recurrence rateOpportunity to treat patients with minimal residual diseaseNo new drug for this patient population in >25 years In 2012 Alone, There Were 73,000 New Cases of Bladder Cancer Reported and 15,000 Deaths *
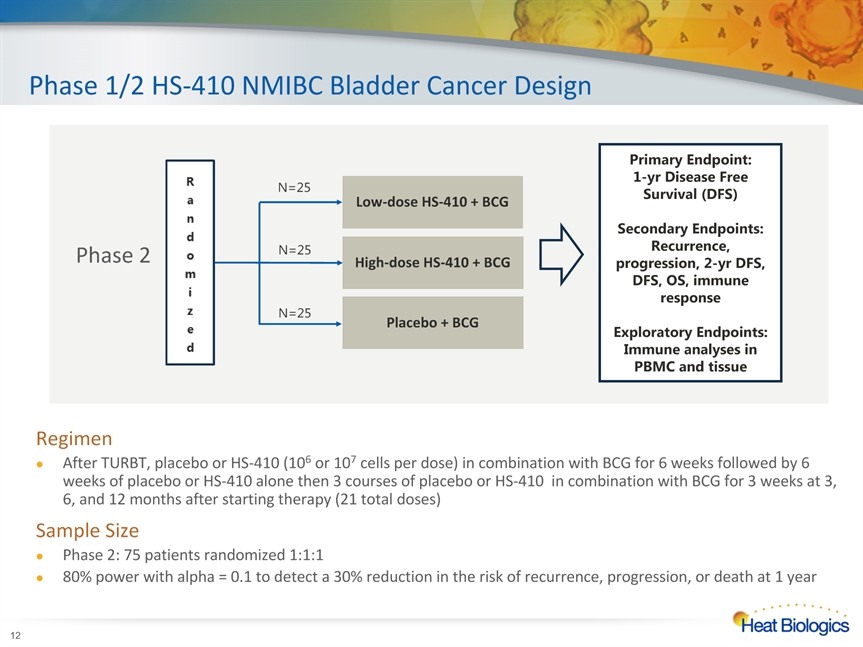
Phase 1/2 HS-410 NMIBC Bladder Cancer Design * RegimenAfter TURBT, placebo or HS-410 (106 or 107 cells per dose) in combination with BCG for 6 weeks followed by 6 weeks of placebo or HS-410 alone then 3 courses of placebo or HS-410 in combination with BCG for 3 weeks at 3, 6, and 12 months after starting therapy (21 total doses)Sample SizePhase 2: 75 patients randomized 1:1:180% power with alpha = 0.1 to detect a 30% reduction in the risk of recurrence, progression, or death at 1 year
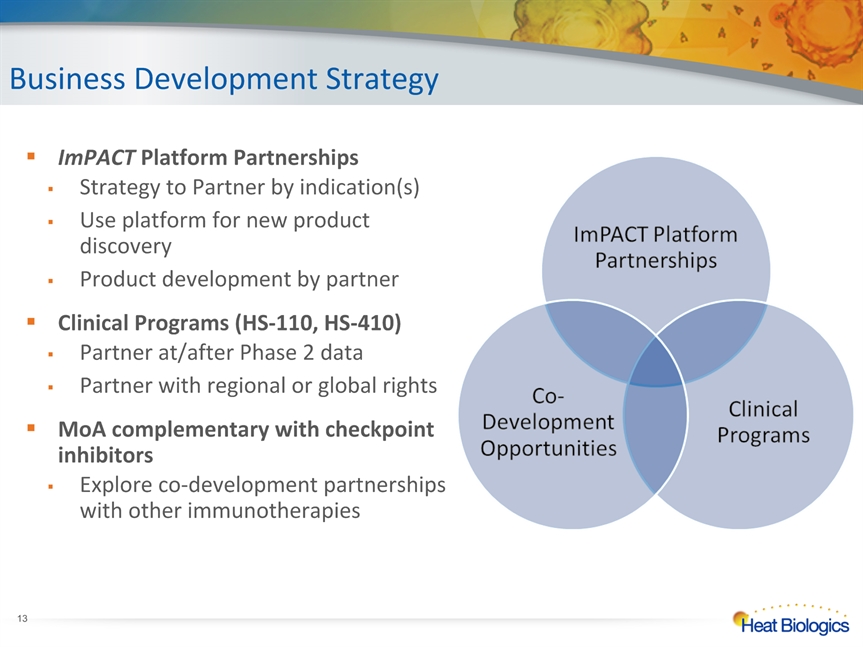
Business Development Strategy * ImPACT Platform PartnershipsStrategy to Partner by indication(s)Use platform for new product discoveryProduct development by partnerClinical Programs (HS-110, HS-410)Partner at/after Phase 2 dataPartner with regional or global rightsMoA complementary with checkpoint inhibitorsExplore co-development partnerships with other immunotherapies
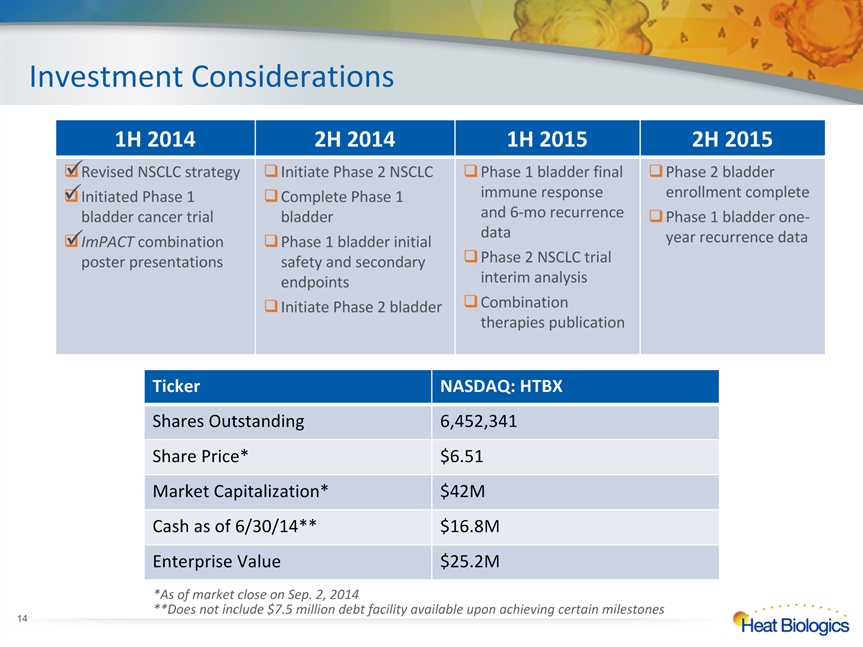
* 1H 2014 2H 2014 1H 2015 2H 2015 Revised NSCLC strategyInitiated Phase 1 bladder cancer trialImPACT combination poster presentations Initiate Phase 2 NSCLC Complete Phase 1 bladder Phase 1 bladder initial safety and secondary endpointsInitiate Phase 2 bladder Phase 1 bladder final immune response and 6-mo recurrence dataPhase 2 NSCLC trial interim analysisCombination therapies publication Phase 2 bladder enrollment completePhase 1 bladder one-year recurrence data Investment Considerations Ticker NASDAQ: HTBX Shares Outstanding 6,452,341 Share Price* $6.51 Market Capitalization* $42M Cash as of 6/30/14** $16.8M Enterprise Value $25.2M *As of market close on Sep. 2, 2014**Does not include $7.5 million debt facility available upon achieving certain milestones
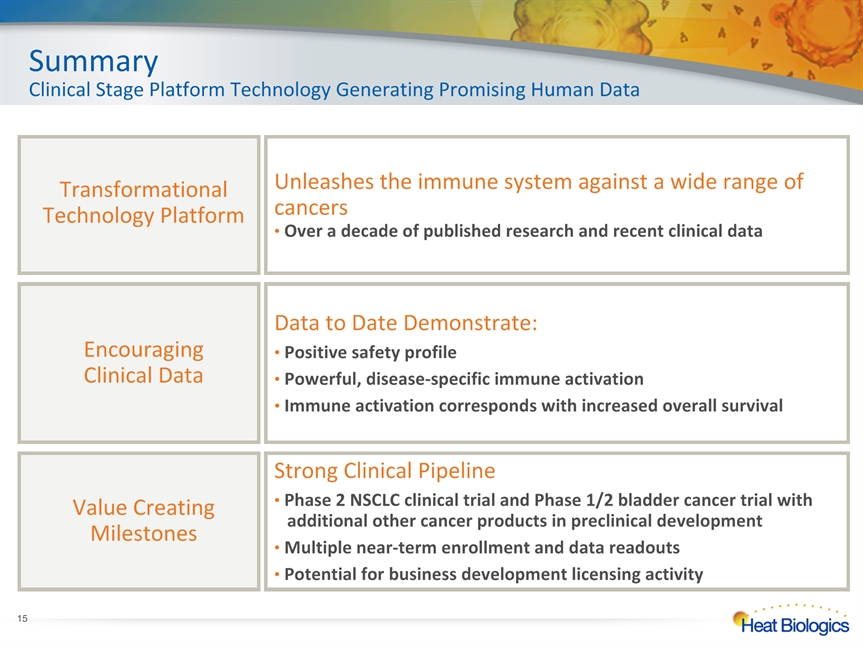
SummaryClinical Stage Platform Technology Generating Promising Human Data Data to Date Demonstrate: Positive safety profile Powerful, disease-specific immune activation Immune activation corresponds with increased overall survival EncouragingClinical Data Strong Clinical Pipeline Phase 2 NSCLC clinical trial and Phase 1/2 bladder cancer trial with additional other cancer products in preclinical development Multiple near-term enrollment and data readouts Potential for business development licensing activity Value Creating Milestones Transformational Technology Platform Unleashes the immune system against a wide range of cancers Over a decade of published research and recent clinical data *
Living Drug Factories to Treat Cancer