PRESS RELEASE
Published on May 29, 2015

Corporate Presentation May 29, 2015 NASDAQ:HTBX

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. *

Overview Heat’s ImPACT allogeneic immunotherapy platform specifically designed to activate patient’s CD8+ cytotoxic T cells against multiple tumor antigensTwo lead programs are actively enrolling patients in randomized, controlled Phase II trials in lung and bladder cancerPositive safety profile with >80 patients dosed in 5 clinical studies with no treatment-related SAE’s, AE’s primarily Gr.1 injection site reactionsClinical evidence validating mechanism of action in two ongoing randomized controlled Phase 2 programs in NSCLC and bladder cancerMultiple near-term value-driving milestones with clinical and pre-clinical milestones expected to be reported at least quarterly for the next two years Allogeneic Immune Pan-Antigen Cytotoxic Therapy “ImPACT” Platform *
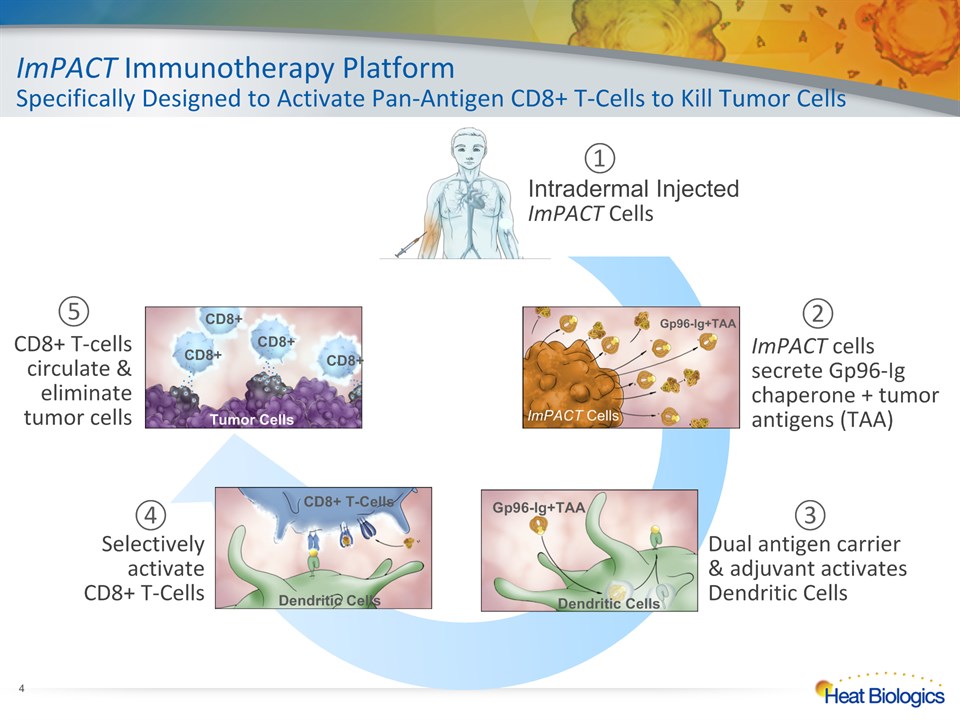
ImPACT Immunotherapy PlatformSpecifically Designed to Activate Pan-Antigen CD8+ T-Cells to Kill Tumor Cells * Intradermal InjectedImPACT Cells ① ImPACT cells secrete Gp96-Ig chaperone + tumor antigens (TAA) ② Dual antigen carrier & adjuvant activates Dendritic Cells ③ Selectively activate CD8+ T-Cells ④ CD8+ T-cellscirculate & eliminate tumor cells ⑤
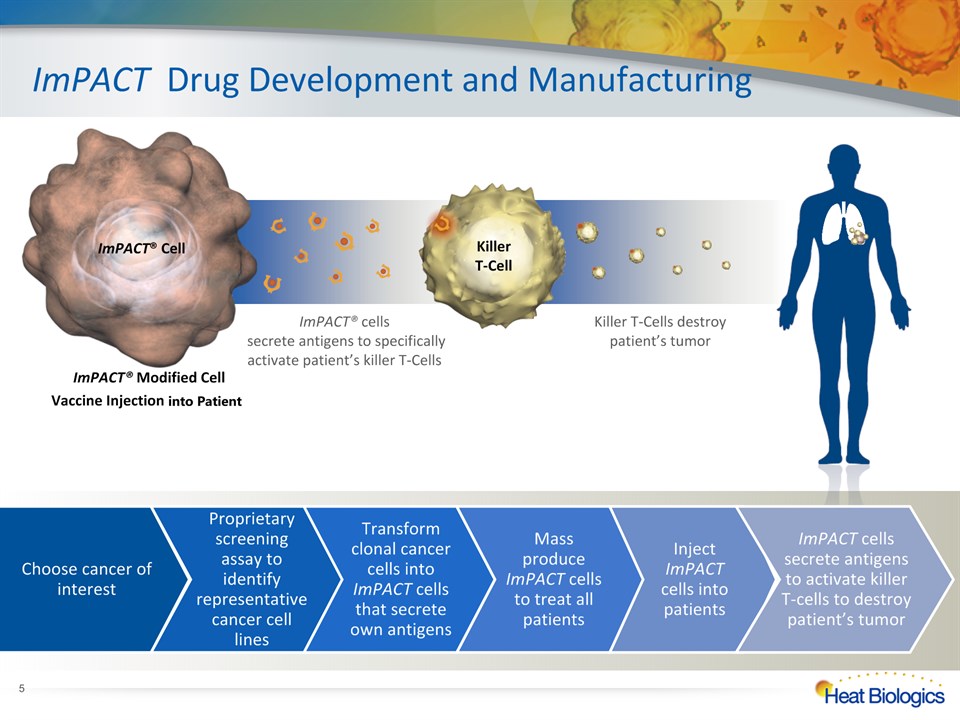
ImPACT® Modified Cell Vaccine Injection into Patient ImPACT Drug Development and Manufacturing ImPACT® Cell Killer T-Cell * Choose cancer of interest Proprietary screening assay to identify representative cancer cell lines Transform clonal cancer cells into ImPACT cells that secrete own antigens Mass produce ImPACT cells to treat all patients Inject ImPACT cells into patients ImPACT cells secrete antigens to activate killer T-cells to destroy patient’s tumor ImPACT® cells secrete antigens to specifically activate patient’s killer T-Cells Killer T-Cells destroy patient’s tumor
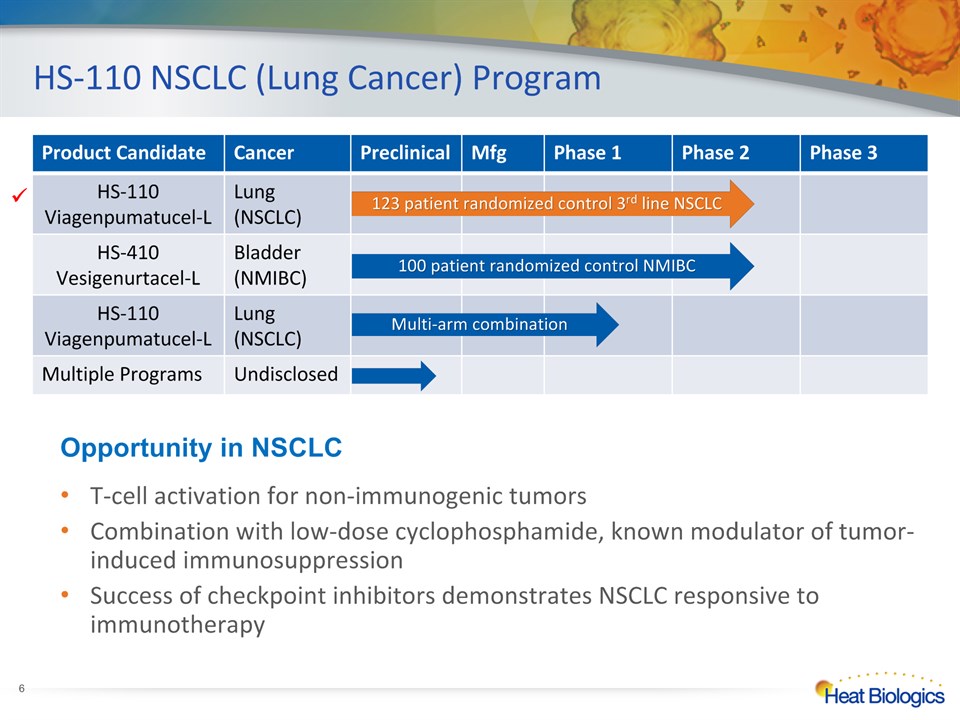
HS-110 NSCLC (Lung Cancer) Program * T-cell activation for non-immunogenic tumorsCombination with low-dose cyclophosphamide, known modulator of tumor-induced immunosuppressionSuccess of checkpoint inhibitors demonstrates NSCLC responsive to immunotherapy Opportunity in NSCLC Product Candidate Cancer Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-110 Viagenpumatucel-L Lung(NSCLC) HS-410Vesigenurtacel-L Bladder(NMIBC) HS-110 Viagenpumatucel-L Lung(NSCLC) Multiple Programs Undisclosed 100 patient randomized control NMIBC Multi-arm combination 123 patient randomized control 3rd line NSCLC 123 patient randomized control 3rd line NSCLC
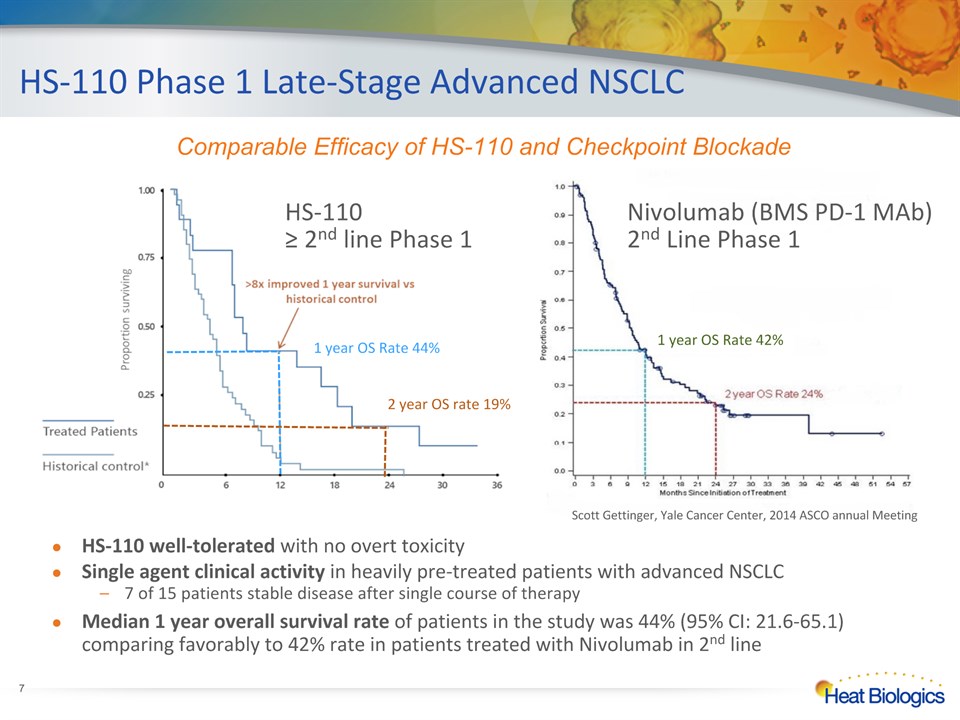
HS-110 Phase 1 Late-Stage Advanced NSCLC * HS-110 ≥ 2nd line Phase 1 HS-110 well-tolerated with no overt toxicity Single agent clinical activity in heavily pre-treated patients with advanced NSCLC7 of 15 patients stable disease after single course of therapy Median 1 year overall survival rate of patients in the study was 44% (95% CI: 21.6-65.1) comparing favorably to 42% rate in patients treated with Nivolumab in 2nd line 1 year OS Rate 42% Nivolumab (BMS PD-1 MAb)2nd Line Phase 1 Comparable Efficacy of HS-110 and Checkpoint Blockade Scott Gettinger, Yale Cancer Center, 2014 ASCO annual Meeting
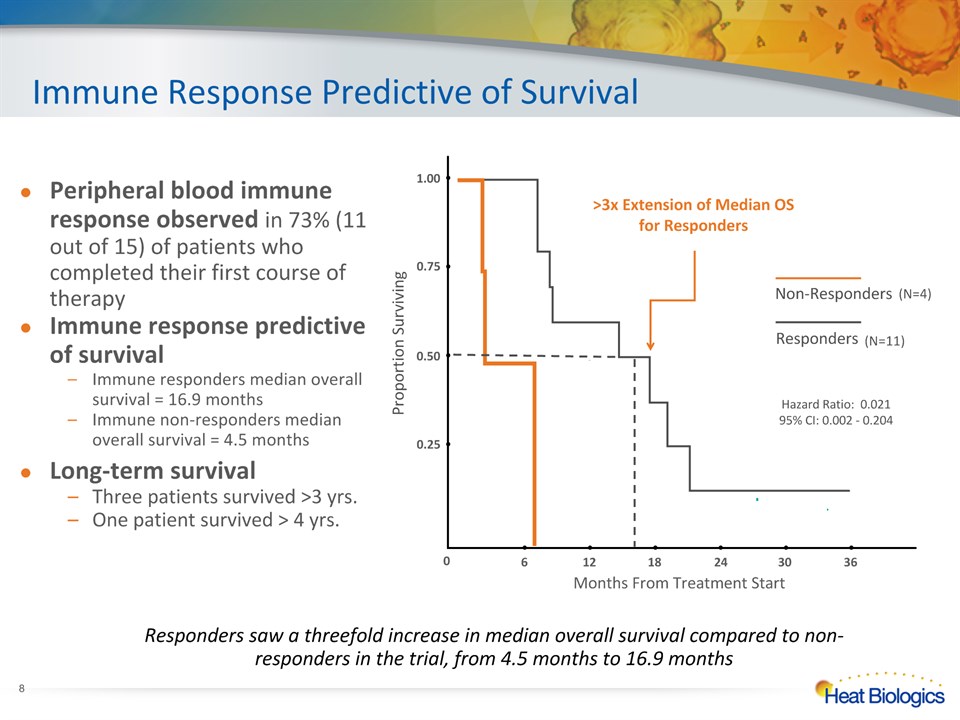
Immune Response Predictive of Survival Responders saw a threefold increase in median overall survival compared to non-responders in the trial, from 4.5 months to 16.9 months Non-Responders Responders >3x Extension of Median OS for Responders 6 12 18 24 30 36 0.25 0.50 1.00 0.75 Proportion Surviving Months From Treatment Start 0 (N=4) (N=11) Hazard Ratio: 0.02195% CI: 0.002 - 0.204 * Peripheral blood immune response observed in 73% (11 out of 15) of patients who completed their first course of therapyImmune response predictive of survival Immune responders median overall survival = 16.9 monthsImmune non-responders median overall survival = 4.5 monthsLong-term survivalThree patients survived >3 yrs.One patient survived > 4 yrs.
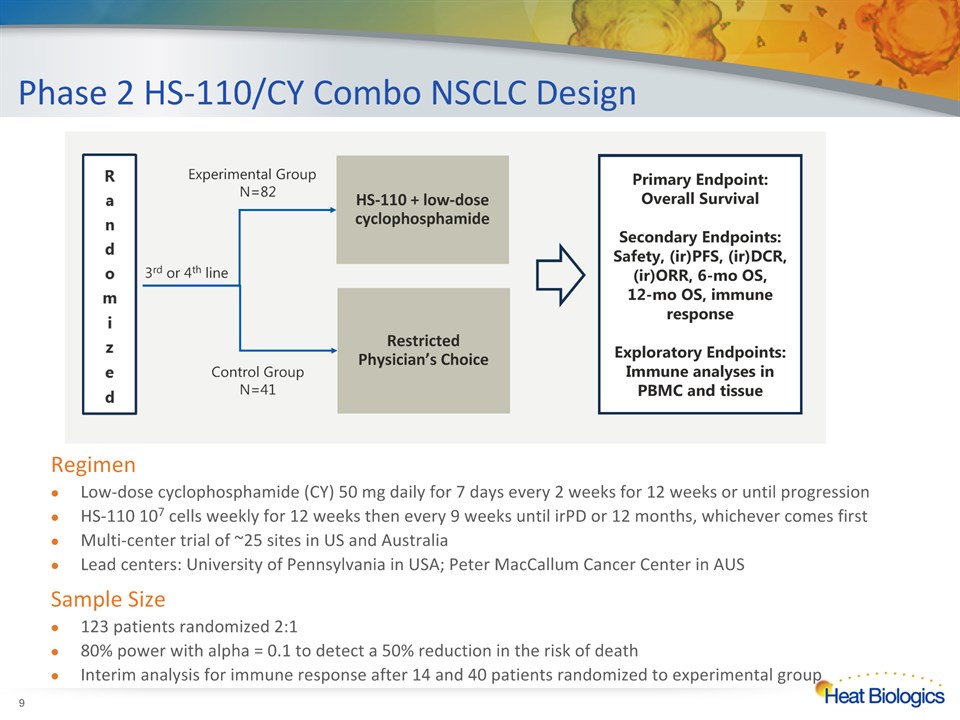
Phase 2 HS-110/CY Combo NSCLC Design * RegimenLow-dose cyclophosphamide (CY) 50 mg daily for 7 days every 2 weeks for 12 weeks or until progressionHS-110 107 cells weekly for 12 weeks then every 9 weeks until irPD or 12 months, whichever comes firstMulti-center trial of ~25 sites in US and AustraliaLead centers: University of Pennsylvania in USA; Peter MacCallum Cancer Center in AUSSample Size123 patients randomized 2:1 80% power with alpha = 0.1 to detect a 50% reduction in the risk of death Interim analysis for immune response after 14 and 40 patients randomized to experimental group 3rd or 4th line
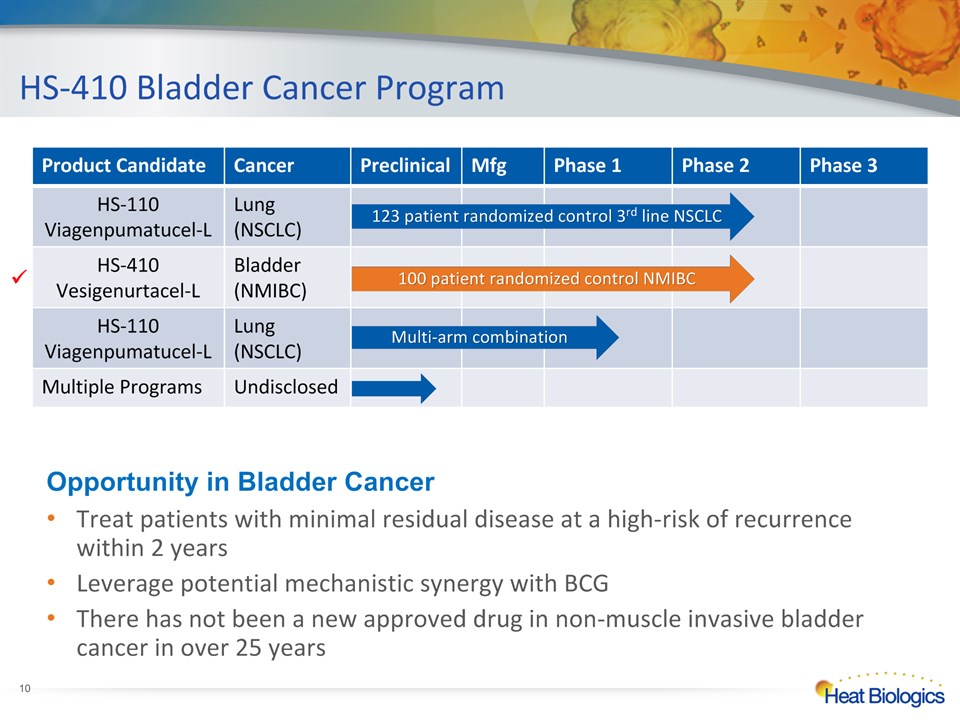
HS-410 Bladder Cancer Program * Treat patients with minimal residual disease at a high-risk of recurrence within 2 yearsLeverage potential mechanistic synergy with BCGThere has not been a new approved drug in non-muscle invasive bladder cancer in over 25 years Product Candidate Cancer Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-110 Viagenpumatucel-L Lung(NSCLC) HS-410Vesigenurtacel-L Bladder(NMIBC) HS-110 Viagenpumatucel-L Lung(NSCLC) Multiple Programs Undisclosed 100 patient randomized control NMIBC Multi-arm combination 123 patient randomized control 3rd line NSCLC Opportunity in Bladder Cancer 100 patient randomized control NMIBC
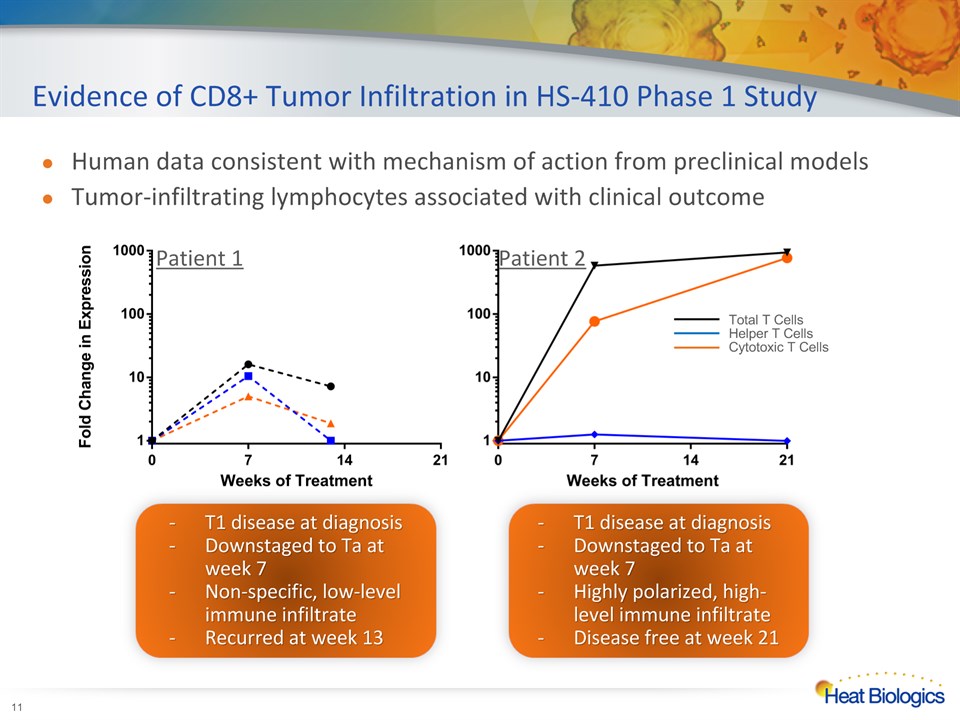
Evidence of CD8+ Tumor Infiltration in HS-410 Phase 1 Study * Patient 1 Patient 2 Total T CellsHelper T CellsCytotoxic T Cells Human data consistent with mechanism of action from preclinical modelsTumor-infiltrating lymphocytes associated with clinical outcome T1 disease at diagnosisDownstaged to Ta at week 7Non-specific, low-level immune infiltrateRecurred at week 13 T1 disease at diagnosisDownstaged to Ta at week 7Highly polarized, high-level immune infiltrateDisease free at week 21
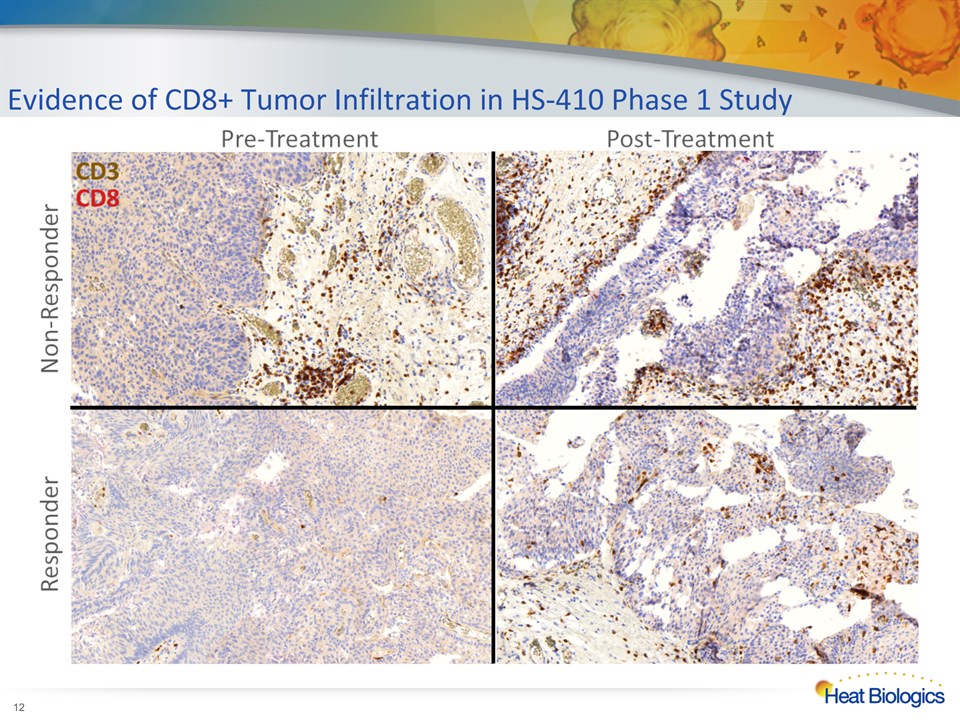
Evidence of CD8+ Tumor Infiltration in HS-410 Phase 1 Study *
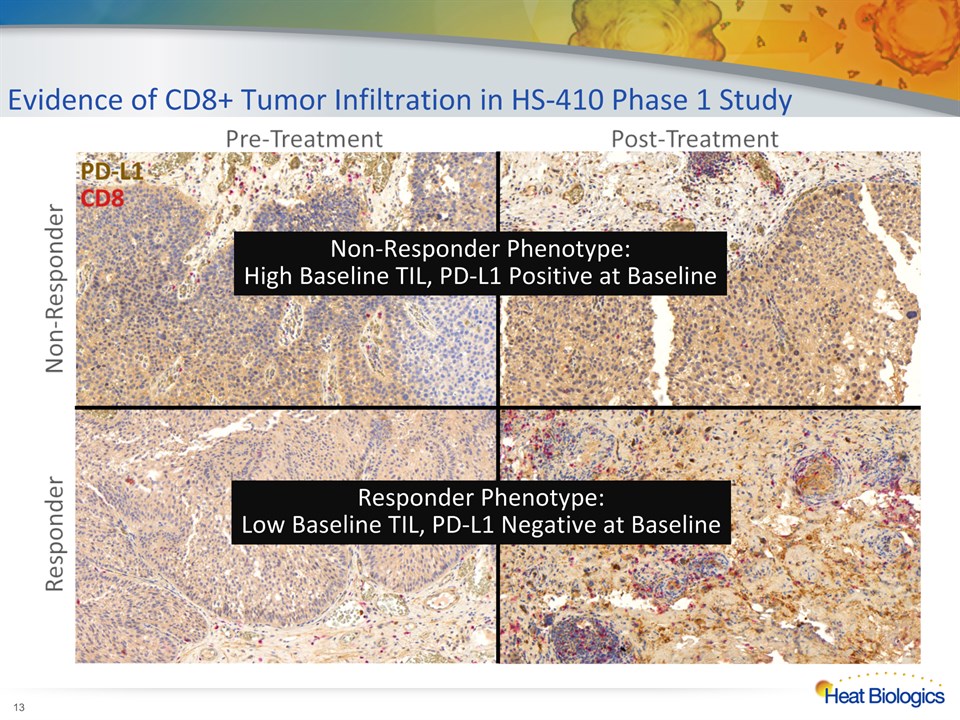
Evidence of CD8+ Tumor Infiltration in HS-410 Phase 1 Study * Non-Responder Phenotype:High Baseline TIL, PD-L1 Positive at Baseline Responder Phenotype:Low Baseline TIL, PD-L1 Negative at Baseline
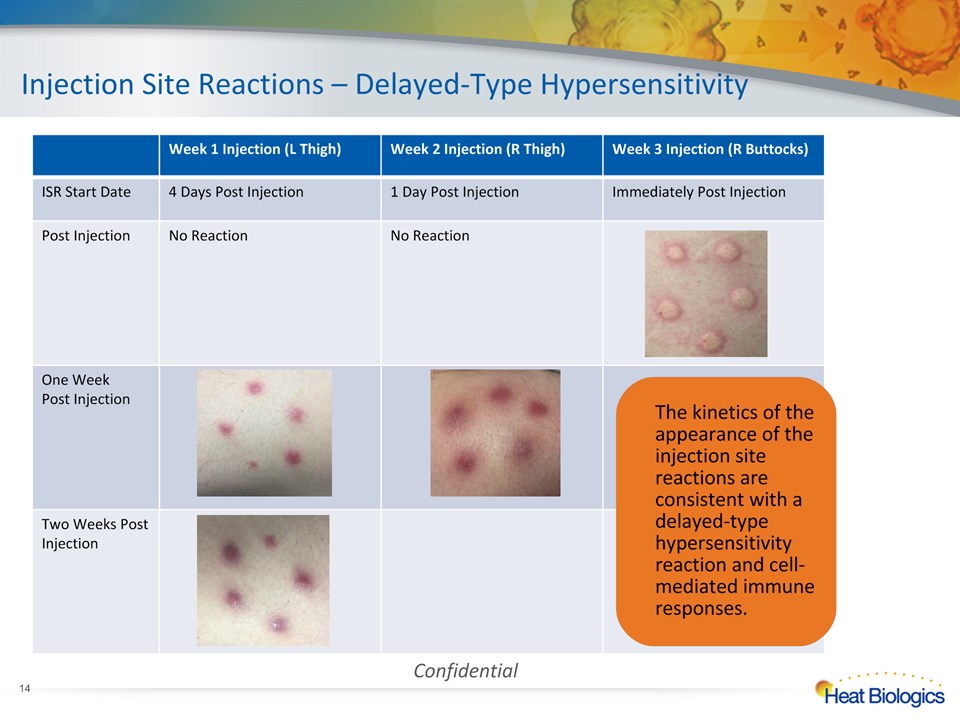
Injection Site Reactions – Delayed-Type Hypersensitivity * Week 1 Injection (L Thigh) Week 2 Injection (R Thigh) Week 3 Injection (R Buttocks) ISR Start Date 4 Days Post Injection 1 Day Post Injection Immediately Post Injection Post Injection No Reaction No Reaction One WeekPost Injection Two Weeks Post Injection Confidential The kinetics of the appearance of the injection site reactions are consistent with a delayed-type hypersensitivity reaction and cell-mediated immune responses.
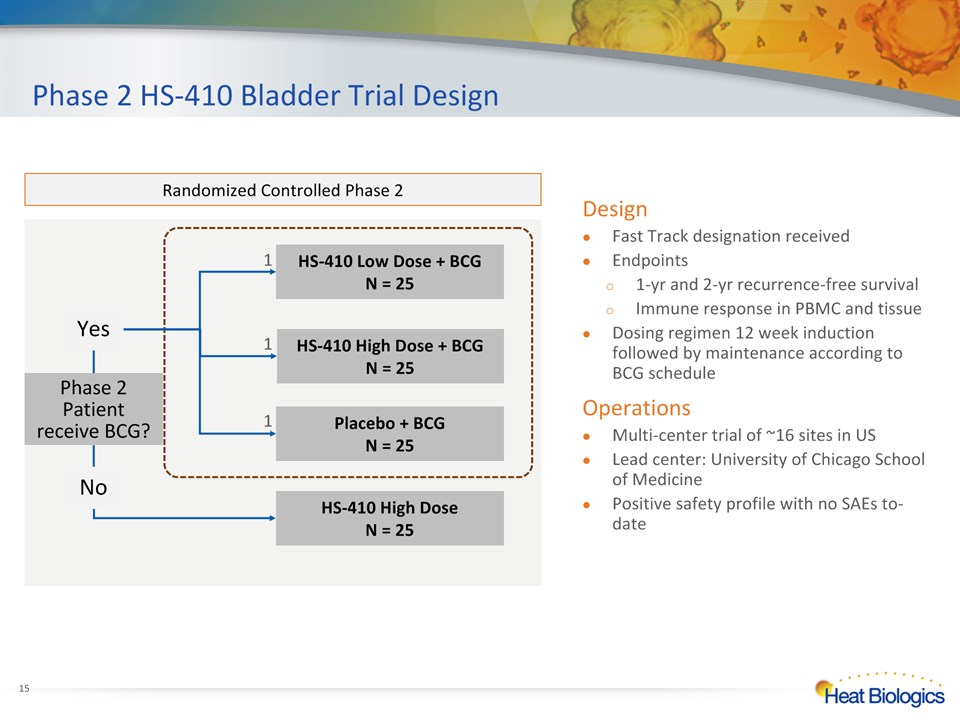
Phase 2 HS-410 Bladder Trial Design * Randomized Controlled Phase 2 HS-410 Low Dose + BCG N = 25 Placebo + BCGN = 25 HS-410 High Dose + BCG N = 25 HS-410 High Dose N = 25 Phase 2 Patient receive BCG? No Yes 1 1 1 DesignFast Track designation receivedEndpoints1-yr and 2-yr recurrence-free survivalImmune response in PBMC and tissueDosing regimen 12 week induction followed by maintenance according to BCG scheduleOperationsMulti-center trial of ~16 sites in USLead center: University of Chicago School of MedicinePositive safety profile with no SAEs to-date

* 2015 Milestones 2nd half of 2015 USA FDA Fast Track Designation Phase 2 Enrollment complete Phase 1 One-year safety and immune response data 1st half of 2015 BladderHS-410 Phase 2 Immune response, first interim analysis Phase 2 - Immune response, second interim analysis- Phase 2 enrollment complete LungHS-110 Multi-Arm Combination Phase 1b Initiate a master multi-arm trial evaluating HS-110 combination with other immunotherapies Phase 1 Preliminary Immune response Additional milestones may include partnership/collaboration opportunities, research developments and new data publications Heat Biologics (Nasdaq: HTBX) gp-96+costimulatory data
ImPACT Clinical Findings Heat’s ImPACT platform is mechanistically differentiated from all other vaccine platforms, providing specific CD8+ T cells activation to multiple tumor antigensPatient tumor biopsies demonstrate specific CD8+ T cell infiltration following treatment, and survival benefit in NSCLC consistent with reported data for PD-1 antibodiesFrequent quarterly reporting of clinical milestones through 2016 *

Heat Biologics (NASDAQ: HTBX): Company Snapshot * *As of May 21, 2015**Does not include $7.5 million milestone-based debt facility Shares Outstanding 8.39M Share Price* ~$6.63 Market Capitalization* ~$51M Cash as of 3/31/15** ~$21M Enterprise Value* ~$30M Heat’s ImPACT allogeneic “off-the-shelf” platform generates robust pan-antigen T cell activation for highly-differentiated mechanism of actionPositive safety profile with >80 patients dosed in 5 clinical studies Clinical evidence validating mechanism of action in two ongoing randomized controlled Phase 2 programs in NSCLC and bladder cancerMultiple near-term value-driving inflection pointsFounded in 2008, completed IPO 2013

Management and Advisors * Jeff WolfFounder & CEO Founded several biomedical companies including Avigen, TyRx Pharma (sold to Medtronic), EluSys Therapeutics, GenerationOne Anil Goyal, Ph.D.VP Business Development 20+ years biotech business development experience: Serenex (acquired by Pfizer), Millennium Pharmaceuticals, Genome Therapeutics, Qualiber, Ascletis Pharmaceuticals, Merck Taylor Schreiber, M.D., Ph.D.VP Research & Development Co-developer ImPACT and TNFRSF25 technologies; 10+ years laboratory experience; Author numerous immunology and immunotherapy abstracts and publications Melissa Price, Ph.D.VP Clinical & Regulatory Affairs 14+ years in oncology clinical development in biotech and CRO space: INC Research, Novaquest, Attenuon Scientific And Clinical Advisors
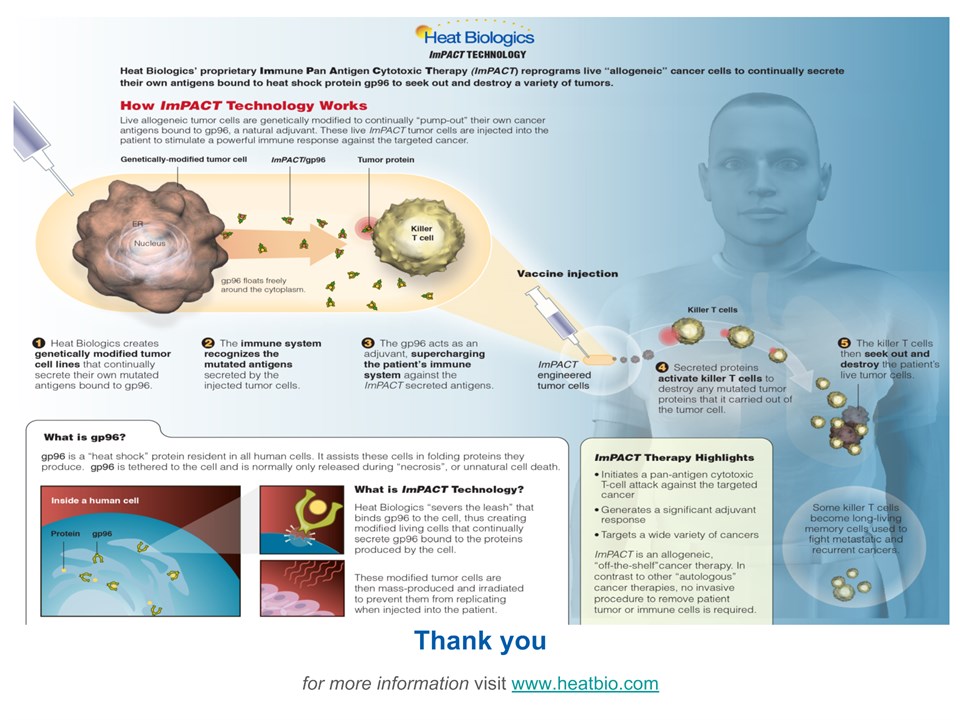
Living Drug Factories to Treat Cancer Thank youfor more information visit www.heatbio.com

Appendix
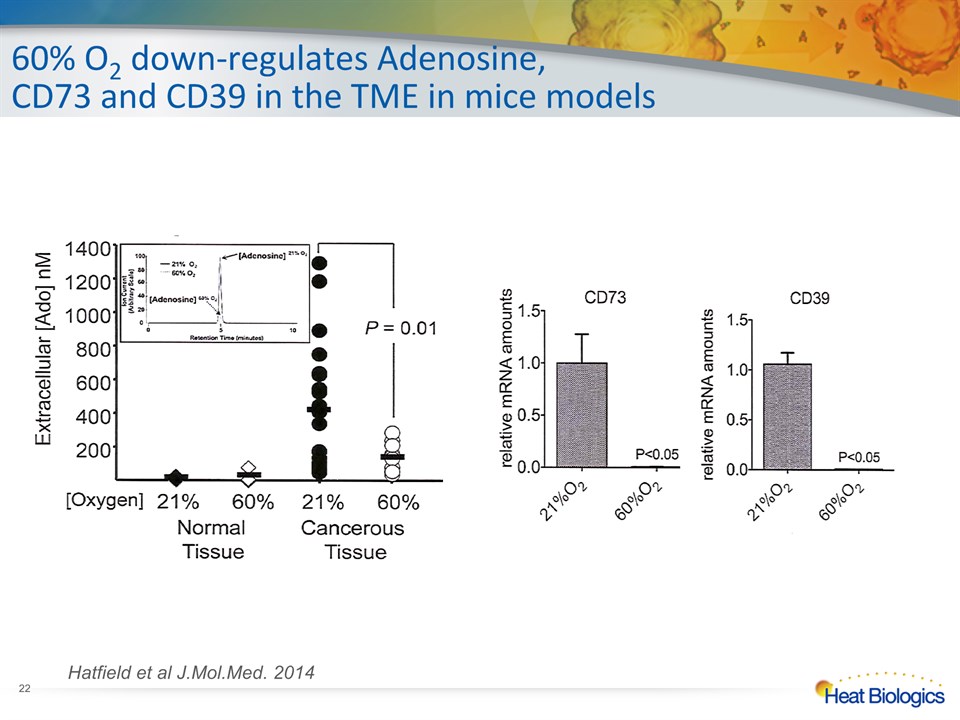
60% O2 down-regulates Adenosine,CD73 and CD39 in the TME in mice models Hatfield et al J.Mol.Med. 2014 *
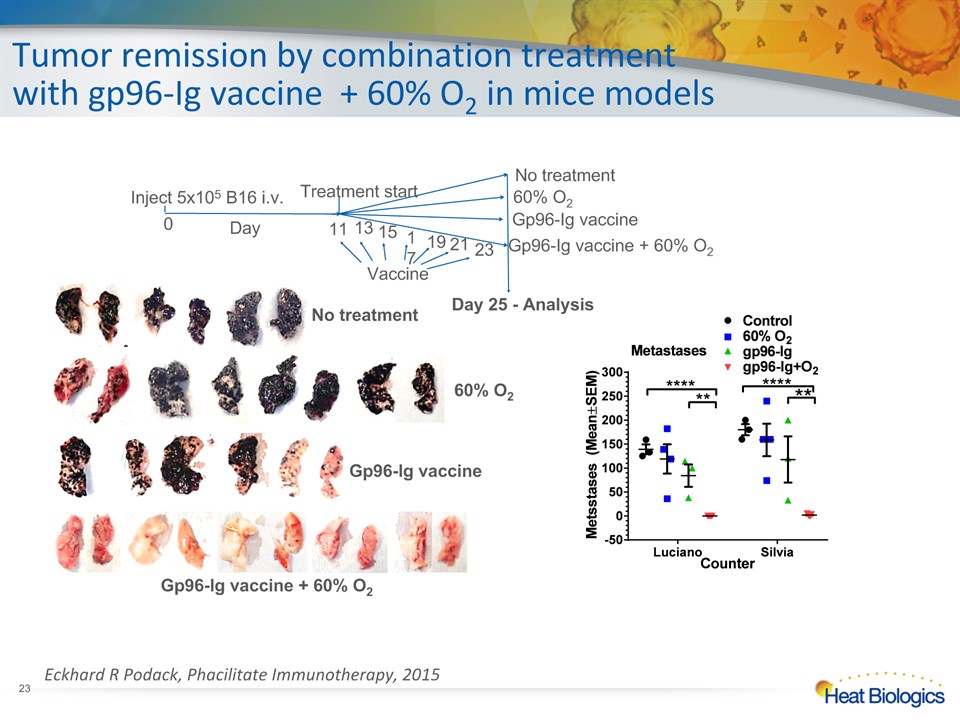
No treatment 60% O2 Gp96-Ig vaccine Gp96-Ig vaccine + 60% O2 Day 25 - Analysis Vaccine * Tumor remission by combination treatment with gp96-Ig vaccine + 60% O2 in mice models Eckhard R Podack, Phacilitate Immunotherapy, 2015
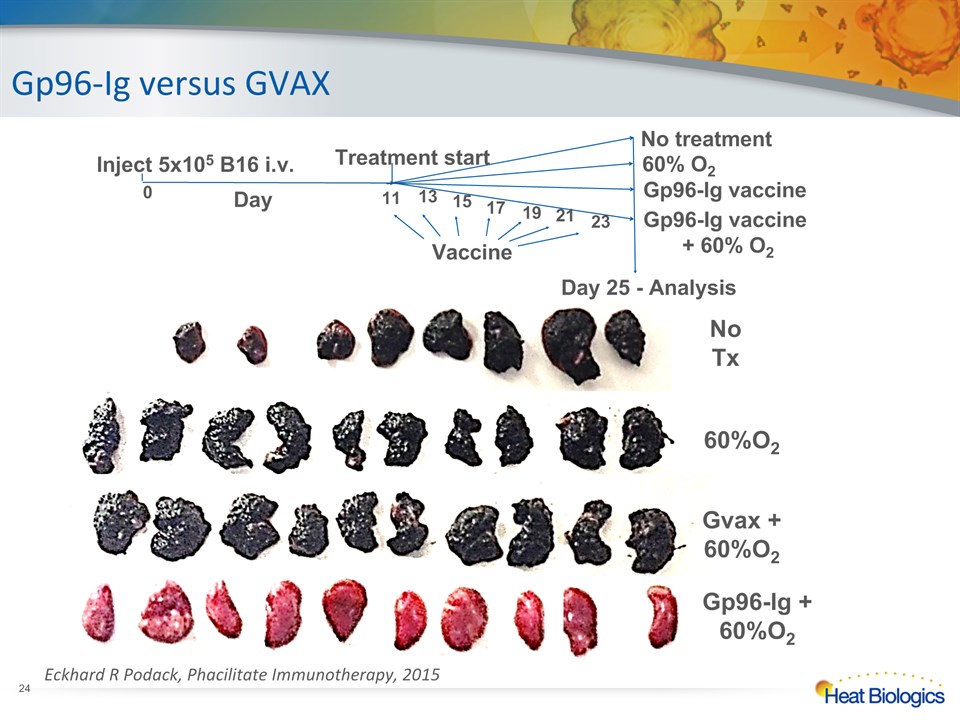
NoTx 60%O2 Gvax +60%O2 Gp96-Ig +60%O2 Day 25 - Analysis Vaccine Gp96-Ig versus GVAX * Eckhard R Podack, Phacilitate Immunotherapy, 2015
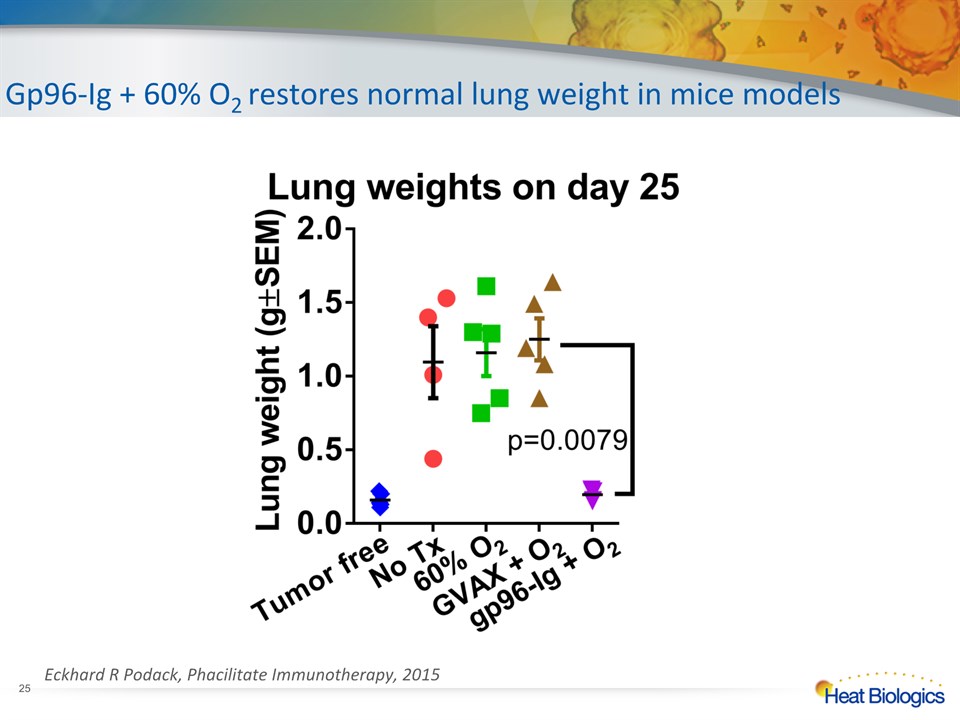
Gp96-Ig + 60% O2 restores normal lung weight in mice models * Eckhard R Podack, Phacilitate Immunotherapy, 2015
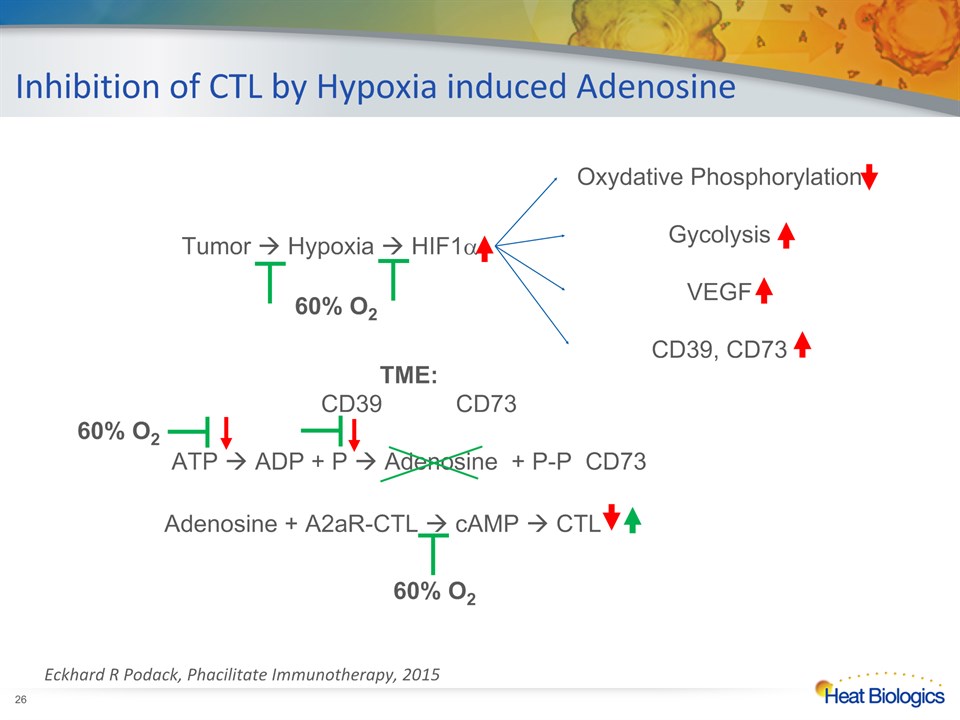
Inhibition of CTL by Hypoxia induced Adenosine Tumor Hypoxia HIF1a Oxydative PhosphorylationGycolysisVEGFCD39, CD73 TME: CD39 CD73ATP ADP + P Adenosine + P-P CD73 Adenosine + A2aR-CTL cAMP CTL 60% O2 60% O2 60% O2 * Eckhard R Podack, Phacilitate Immunotherapy, 2015