POWER POINT PRESENTATION
Published on November 9, 2015
EXHIBIT 99.1

NASDAQ:HTBX November 6, 2015

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2
Investment Opportunity 3 Developing first new immunotherapy in non-muscle invasive bladder cancer (NMIBC) Platform technologies designed to activate CD8+ T cells against multiple tumor antigens Immuno-oncology company developing novel therapies to activate a patient’s immune system against cancer Conducting first vaccine + PD-1 checkpoint inhibitor combo trial in non-small cell lung cancer (NSCLC) Clinical evidence of mechanism of actionIncreased CD8+ T cells in tumors associated with clinical response
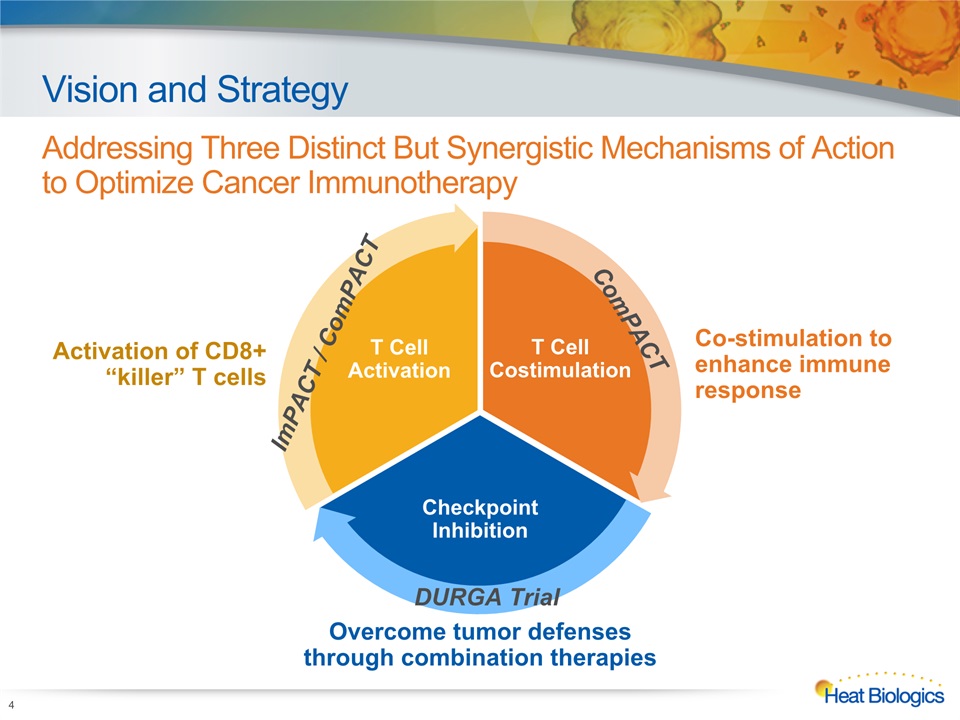
Vision and Strategy Addressing Three Distinct But Synergistic Mechanisms of Action to Optimize Cancer Immunotherapy 4 Activation of CD8+ “killer” T cells Co-stimulation to enhance immune response Overcome tumor defenses through combination therapies T Cell Costimulation Checkpoint Inhibition T Cell Activation ComPACT ImPACT / ComPACT DURGA Trial
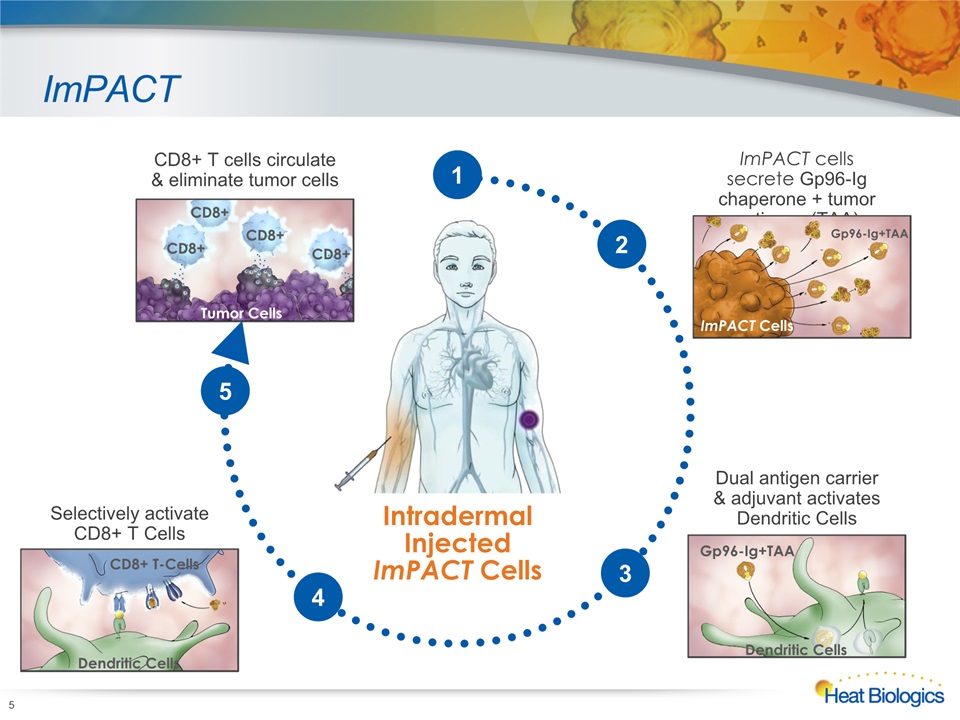
ImPACT 5 Intradermal InjectedImPACT Cells ImPACT cells secrete Gp96-Ig chaperone + tumor antigens (TAA) ImPACT Cells Gp96-Ig+TAA Selectively activate CD8+ T Cells CD8+ T-Cells Dendritic Cells CD8+ T cells circulate & eliminate tumor cells Tumor Cells CD8+ CD8+ CD8+ CD8+ 2 Dual antigen carrier & adjuvant activates Dendritic Cells Gp96-Ig+TAA Dendritic Cells 3 4 5 1
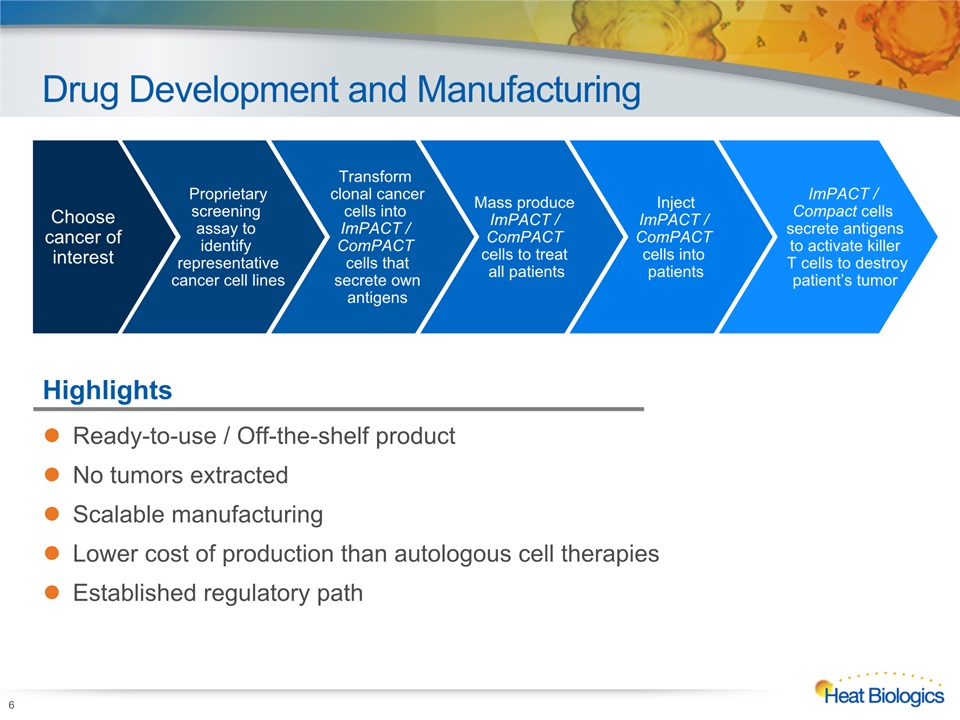
Drug Development and Manufacturing 6 Highlights Ready-to-use / Off-the-shelf productNo tumors extractedScalable manufacturingLower cost of production than autologous cell therapiesEstablished regulatory path Choose cancer of interest Proprietaryscreening assay to identify representativecancer cell lines Transform clonal cancercells into ImPACT / ComPACT cells thatsecrete ownantigens Mass produce ImPACT / ComPACT cells to treat all patients InjectImPACT / ComPACT cells into patients ImPACT / Compact cells secrete antigensto activate killer T cells to destroypatient’s tumor
ComPACT 7 gp96-Ig OX40L-Fc The first potential dual-acting immunotherapy designed to deliver T cell activation and costimulation in a single product
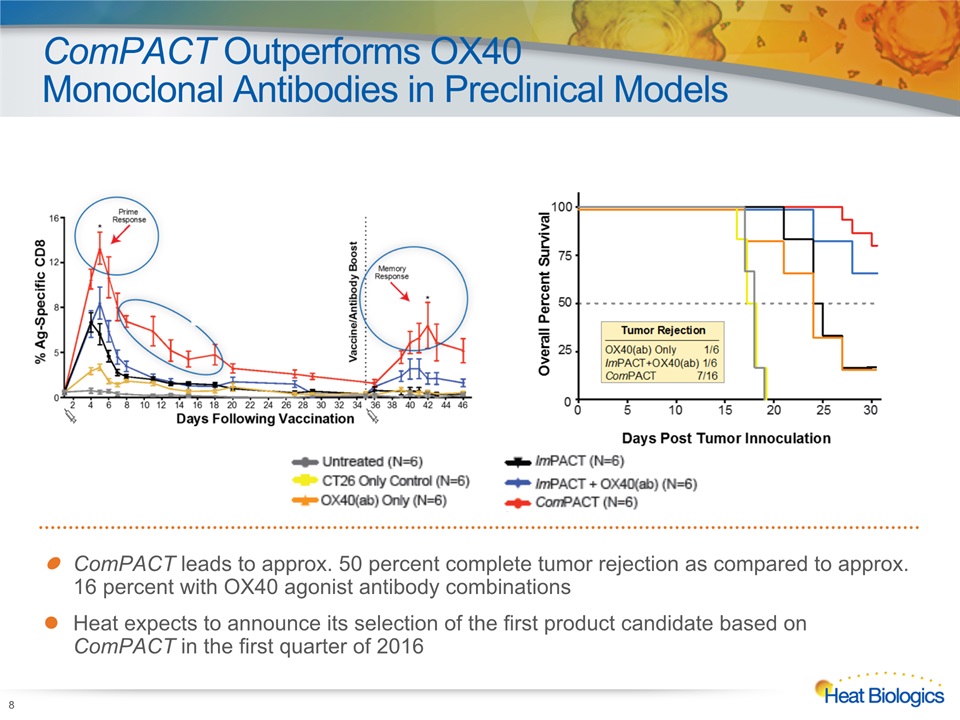
ComPACT Outperforms OX40 Monoclonal Antibodies in Preclinical Models 8 ComPACT leads to approx. 50 percent complete tumor rejection as compared to approx. 16 percent with OX40 agonist antibody combinationsHeat expects to announce its selection of the first product candidate based on ComPACT in the first quarter of 2016
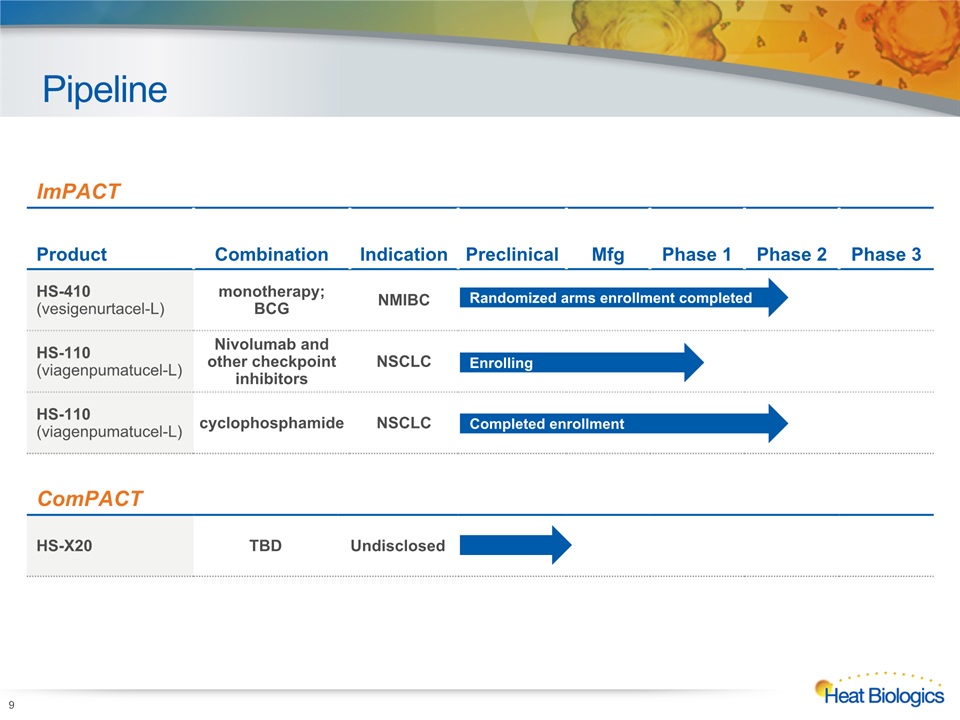
Pipeline 9 Heat Biologics Corporate Model Deck ImPACT Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) monotherapy; BCG NMIBC HS-110(viagenpumatucel-L) Nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC ComPACT HS-X20 TBD Undisclosed Randomized arms enrollment completed Enrolling Completed enrollment

Pipeline 10 Heat Biologics Corporate Model Deck ImPACT Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) monotherapy; BCG NMIBC HS-110(viagenpumatucel-L) Nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC ComPACT HS-X20 TBD Undisclosed Randomized arms enrollment completed Enrolling Completed enrollment HS-410 received fast track designation from U.S. FDA
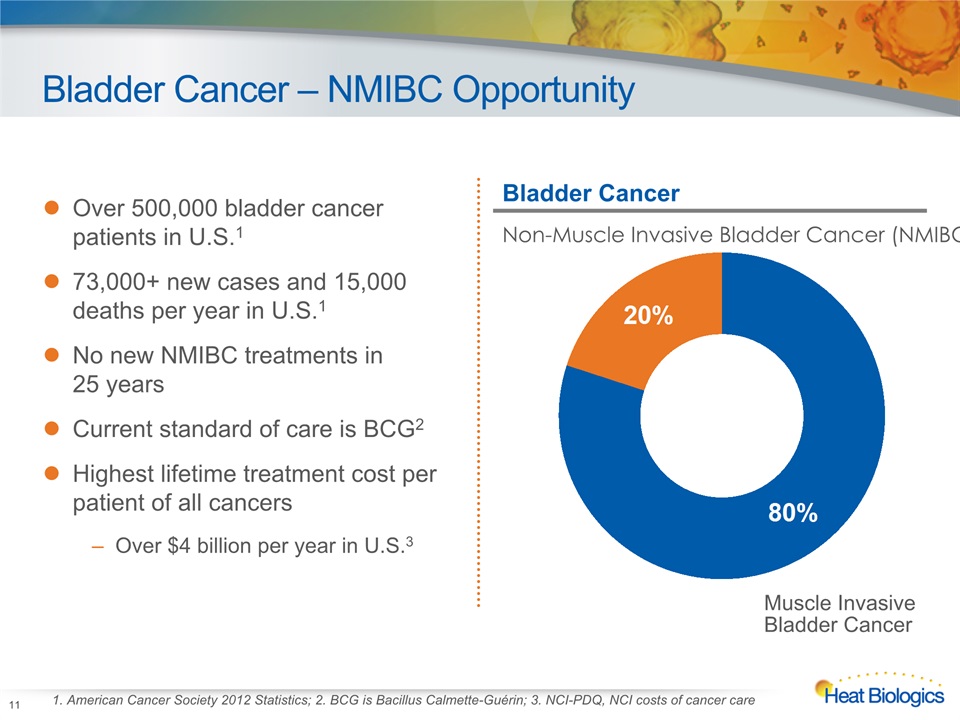
Bladder Cancer – NMIBC Opportunity 1. American Cancer Society 2012 Statistics; 2. BCG is Bacillus Calmette-Guérin; 3. NCI-PDQ, NCI costs of cancer care Over 500,000 bladder cancer patients in U.S.173,000+ new cases and 15,000 deaths per year in U.S.1No new NMIBC treatments in 25 yearsCurrent standard of care is BCG2Highest lifetime treatment cost per patient of all cancers Over $4 billion per year in U.S.3 Bladder Cancer Non-Muscle Invasive Bladder Cancer (NMIBC) 11 Muscle InvasiveBladder Cancer
HS-410 Phase 1 NMIBC Trial Overview Design Open-label, multicenter safety trial; 10 patients enrolledPatients received BCG for 3-6 weeks followed by weekly intradermal injections of HS-410 for 12 weeks and subsequent monthly HS-410 injections for 3 months Results HS-410 had a positive safety profile and was well-tolerated; no serious adverse events or treatment discontinuations7 out of 10 patients had no documented recurrences >1 year after standard of care surgeryUnprecedented increase in intratumoral CD8+ T cells following vaccination Broad-based (polyclonal) expansion of patient T cellsHS-410 shared 15 or more tumor antigens in common with those expressed on the patients’ cancer cellsStrong correlation between baseline characteristics of TILs by T cell receptor (TCR) sequencing and clinical outcome 12
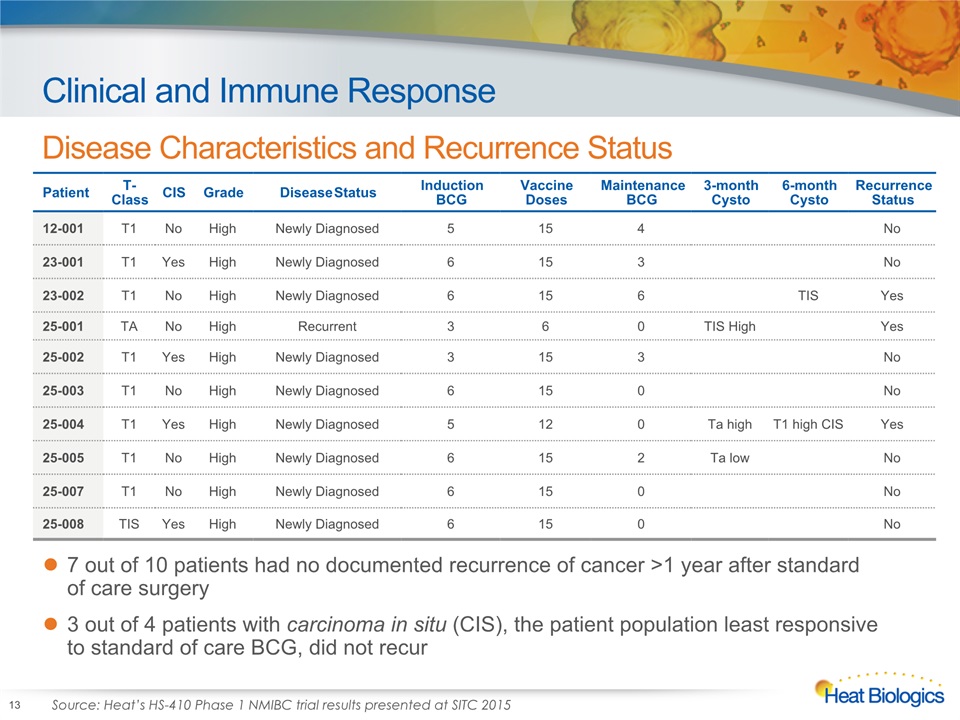
Clinical and Immune Response Disease Characteristics and Recurrence Status 13 Patient T-Class CIS Grade Disease Status Induction BCG Vaccine Doses Maintenance BCG 3-month Cysto 6-month Cysto Recurrence Status 12-001 T1 No High Newly Diagnosed 5 15 4 No 23-001 T1 Yes High Newly Diagnosed 6 15 3 No 23-002 T1 No High Newly Diagnosed 6 15 6 TIS Yes 25-001 TA No High Recurrent 3 6 0 TIS High Yes 25-002 T1 Yes High Newly Diagnosed 3 15 3 No 25-003 T1 No High Newly Diagnosed 6 15 0 No 25-004 T1 Yes High Newly Diagnosed 5 12 0 Ta high T1 high CIS Yes 25-005 T1 No High Newly Diagnosed 6 15 2 Ta low No 25-007 T1 No High Newly Diagnosed 6 15 0 No 25-008 TIS Yes High Newly Diagnosed 6 15 0 No 7 out of 10 patients had no documented recurrence of cancer >1 year after standard of care surgery3 out of 4 patients with carcinoma in situ (CIS), the patient population least responsive to standard of care BCG, did not recur Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015
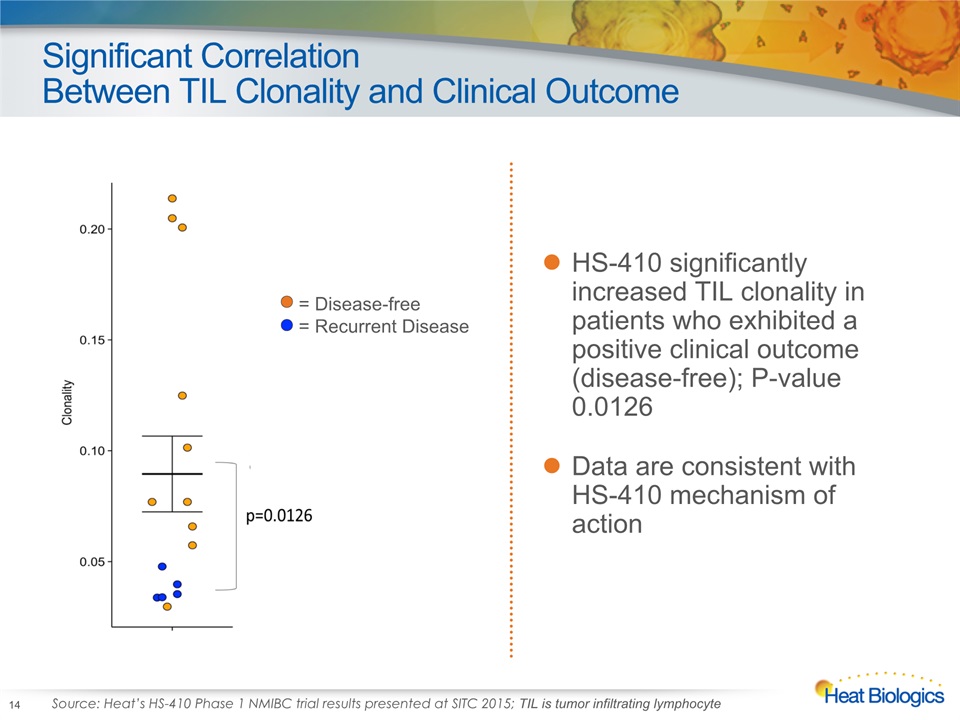
Significant CorrelationBetween TIL Clonality and Clinical Outcome 14 HS-410 significantly increased TIL clonality in patients who exhibited a positive clinical outcome (disease-free); P-value 0.0126 Data are consistent with HS-410 mechanism of action = Disease-free = Recurrent Disease Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015; TIL is tumor infiltrating lymphocyte
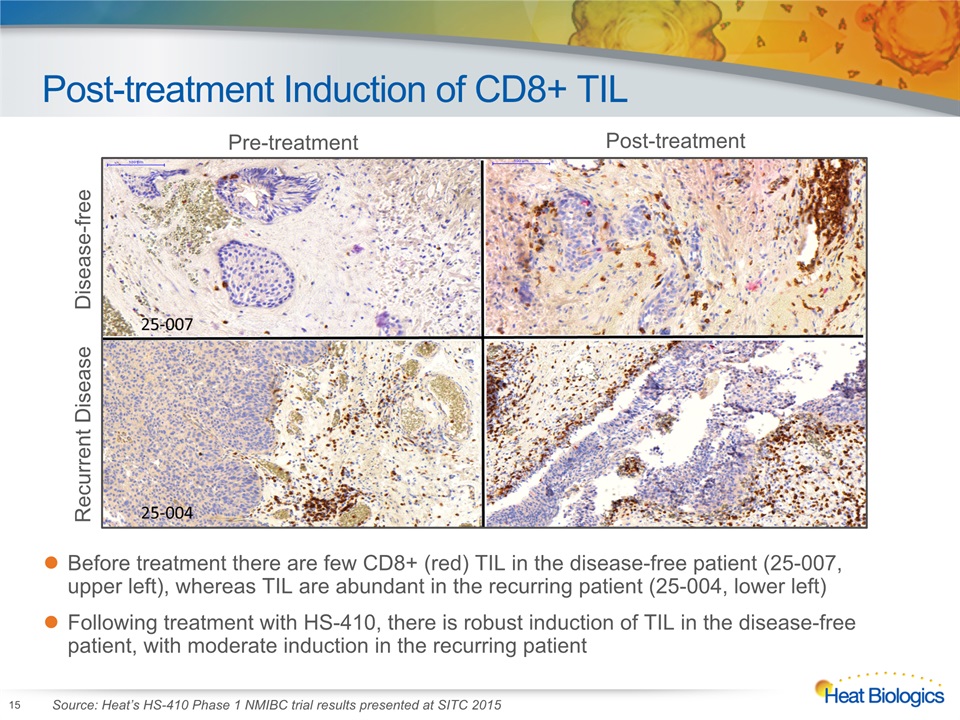
Post-treatment Induction of CD8+ TIL 15 Before treatment there are few CD8+ (red) TIL in the disease-free patient (25-007, upper left), whereas TIL are abundant in the recurring patient (25-004, lower left)Following treatment with HS-410, there is robust induction of TIL in the disease-free patient, with moderate induction in the recurring patient Pre-treatment Post-treatment Recurrent Disease Disease-free Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015
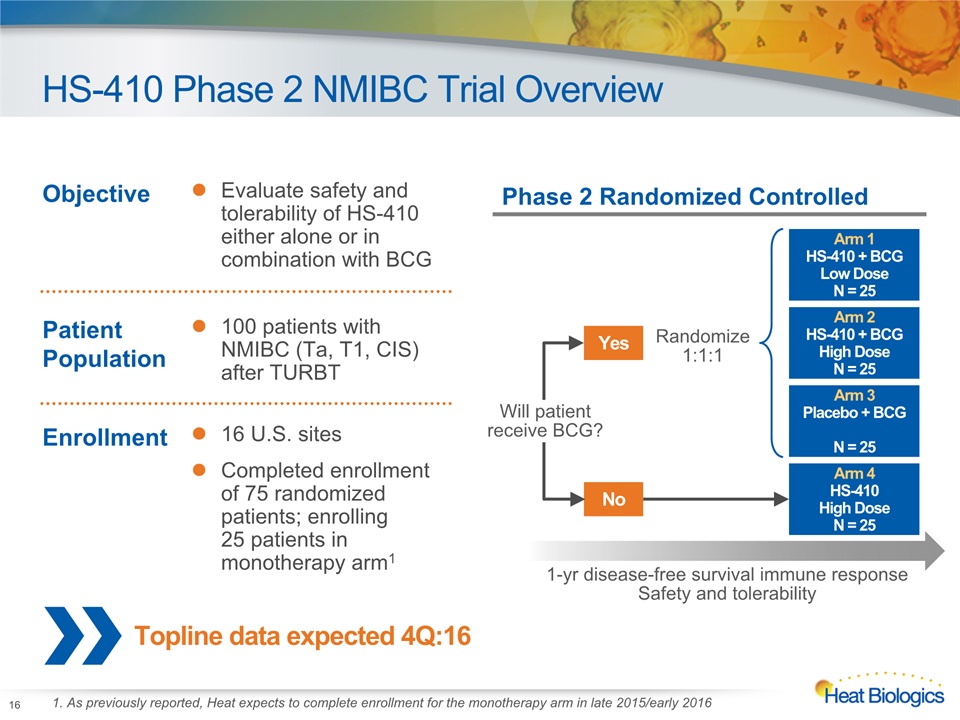
HS-410 Phase 2 NMIBC Trial Overview 16 Topline data expected 4Q:16 Objective Evaluate safety and tolerability of HS-410 either alone or in combination with BCG Patient Population 100 patients with NMIBC (Ta, T1, CIS) after TURBT Enrollment 16 U.S. sitesCompleted enrollment of 75 randomized patients; enrolling 25 patients in monotherapy arm1 Phase 2 Randomized Controlled Arm 2HS-410 + BCGHigh DoseN = 25 Arm 1HS-410 + BCGLow DoseN = 25 Arm 3Placebo + BCGN = 25 Arm 4HS-410 High DoseN = 25 Yes Randomize1:1:1 No Will patientreceive BCG? 1-yr disease-free survival immune responseSafety and tolerability 1. As previously reported, Heat expects to complete enrollment for the monotherapy arm in late 2015/early 2016
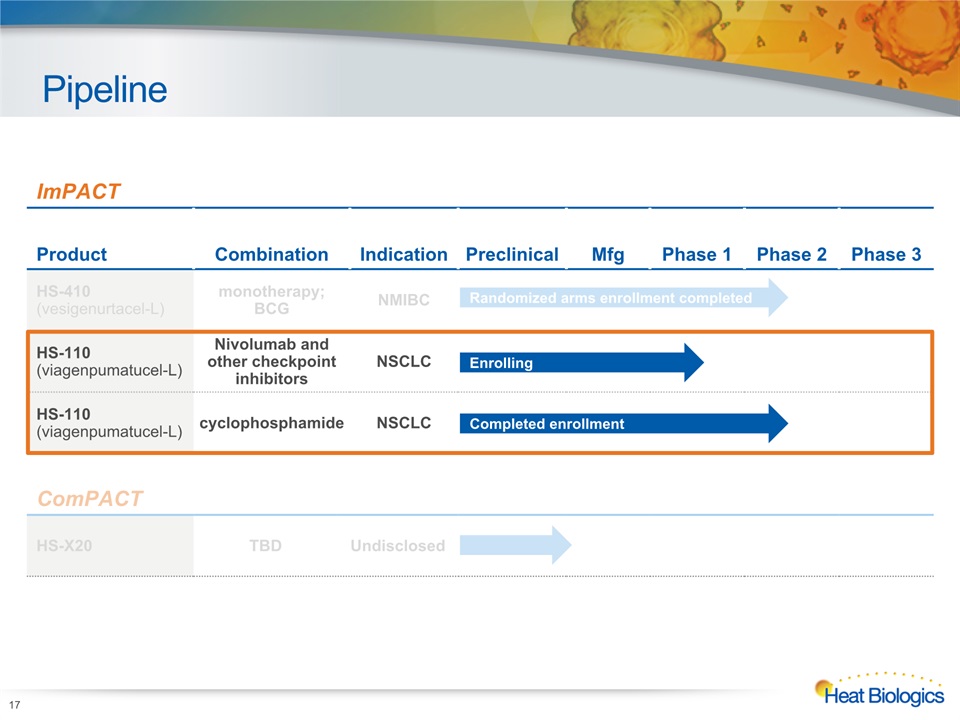
Pipeline 17 Heat Biologics Corporate Model Deck ImPACT Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) monotherapy; BCG NMIBC HS-110(viagenpumatucel-L) Nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC ComPACT HS-X20 TBD Undisclosed Randomized arms enrollment completed Enrolling Completed enrollment
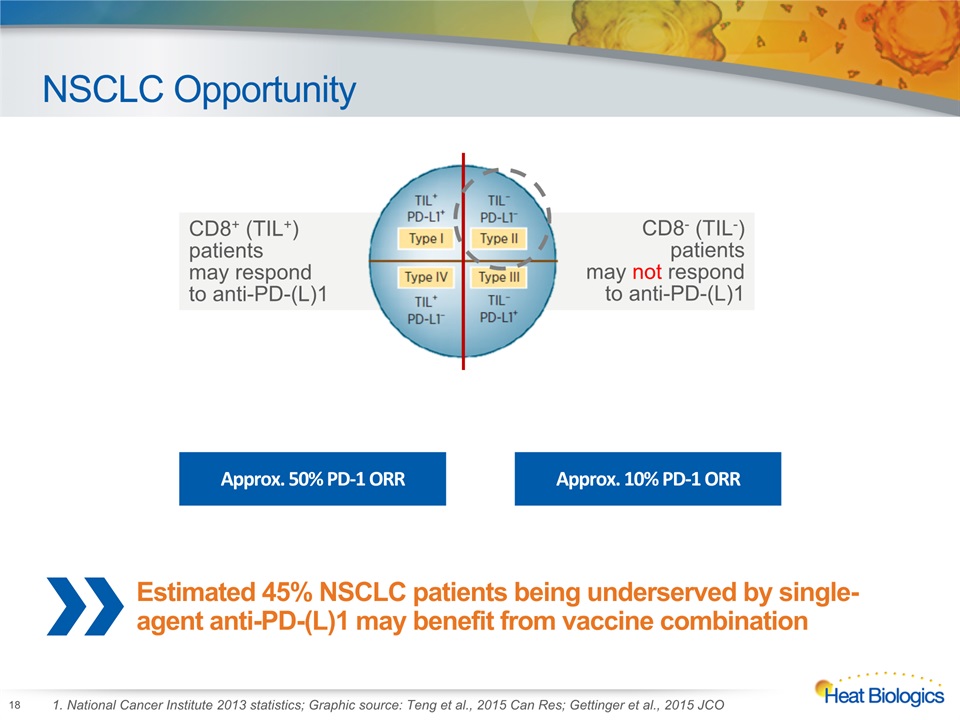
NSCLC Opportunity 18 1. National Cancer Institute 2013 statistics; Graphic source: Teng et al., 2015 Can Res; Gettinger et al., 2015 JCO CD8+ (TIL+) patients may respond to anti-PD-(L)1 CD8- (TIL-) patients may not respond to anti-PD-(L)1 Approx. 50% PD-1 ORR Approx. 10% PD-1 ORR Estimated 45% NSCLC patients being underserved by single-agent anti-PD-(L)1 may benefit from vaccine combination
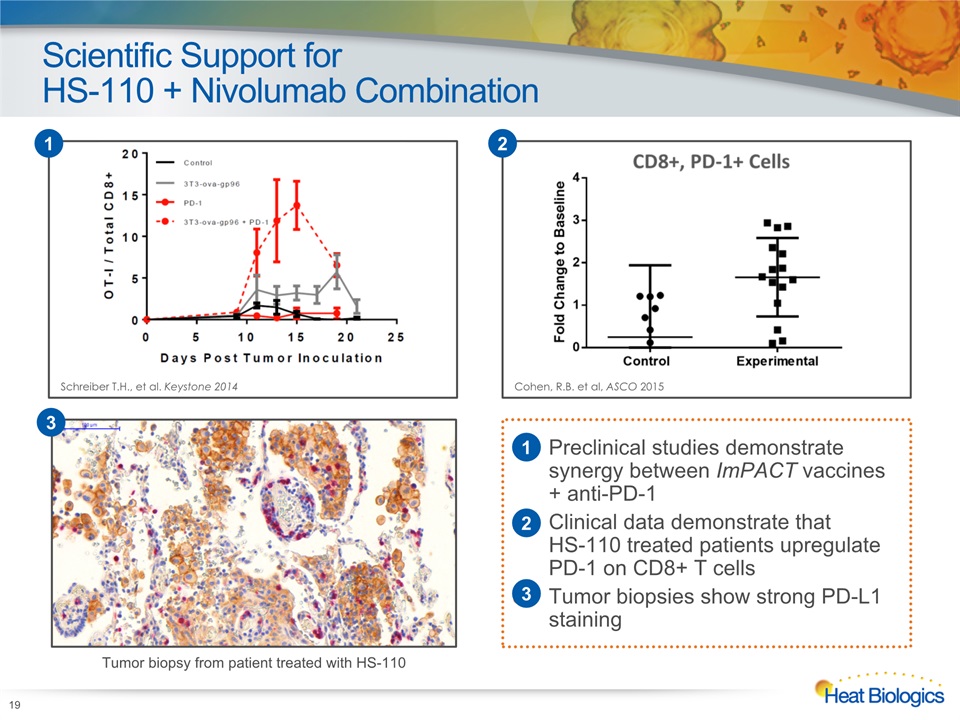
Scientific Support forHS-110 + Nivolumab Combination 19 Cohen, R.B. et al, ASCO 2015 2 Schreiber T.H., et al. Keystone 2014 1 Preclinical studies demonstrate synergy between ImPACT vaccines + anti-PD-1Clinical data demonstrate that HS-110 treated patients upregulate PD-1 on CD8+ T cellsTumor biopsies show strong PD-L1 staining Tumor biopsy from patient treated with HS-110 3 1 2 3
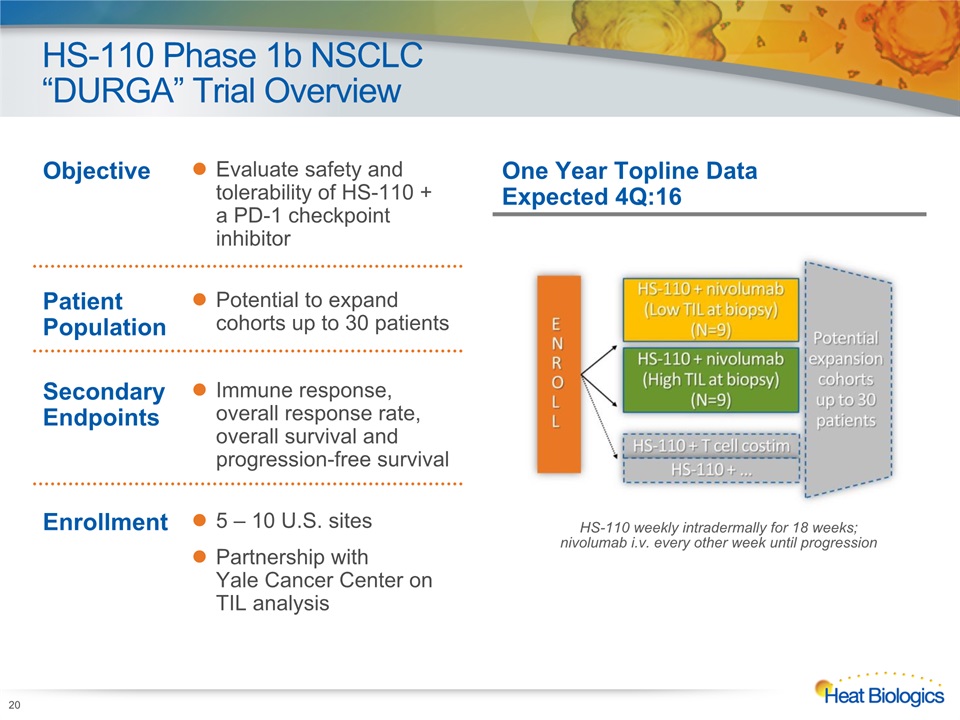
HS-110 Phase 1b NSCLC “DURGA” Trial Overview HS-110 weekly intradermally for 18 weeks; nivolumab i.v. every other week until progression One Year Topline Data Expected 4Q:16 Objective Evaluate safety and tolerability of HS-110 + a PD-1 checkpoint inhibitor Patient Population Potential to expand cohorts up to 30 patients Secondary Endpoints Immune response, overall response rate, overall survival and progression-free survival Enrollment 5 – 10 U.S. sitesPartnership with Yale Cancer Center on TIL analysis 20
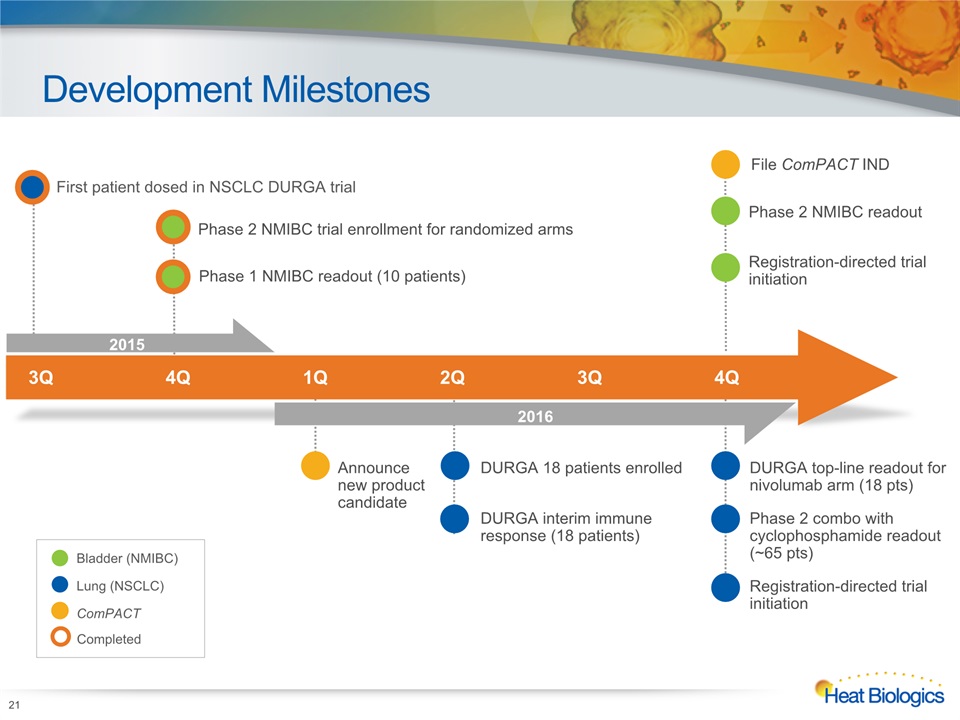
Development Milestones 21 Phase 2 NMIBC trial enrollment for randomized arms DURGA top-line readout for nivolumab arm (18 pts)Phase 2 combo with cyclophosphamide readout (~65 pts)Registration-directed trial initiation Phase 1 NMIBC readout (10 patients) Phase 2 NMIBC readoutRegistration-directed trial initiation DURGA 18 patients enrolledDURGA interim immune response (18 patients) Announce new product candidate Bladder (NMIBC) Lung (NSCLC) ComPACT 3Q 4Q 1Q 2Q 3Q 4Q First patient dosed in NSCLC DURGA trial 2015 2016 Completed File ComPACT IND
Summary: Value Proposition 22 Commercially Viable Combinable with Checkpoints + Others Pan-antigen cell-basedSpecific tumor killingT cell activatingApplicable to all cancers Positive safety profile to dateEstablished regulatory path Ready-to-useScalable, low cost manufacturingNo tumors extracted Differentiated
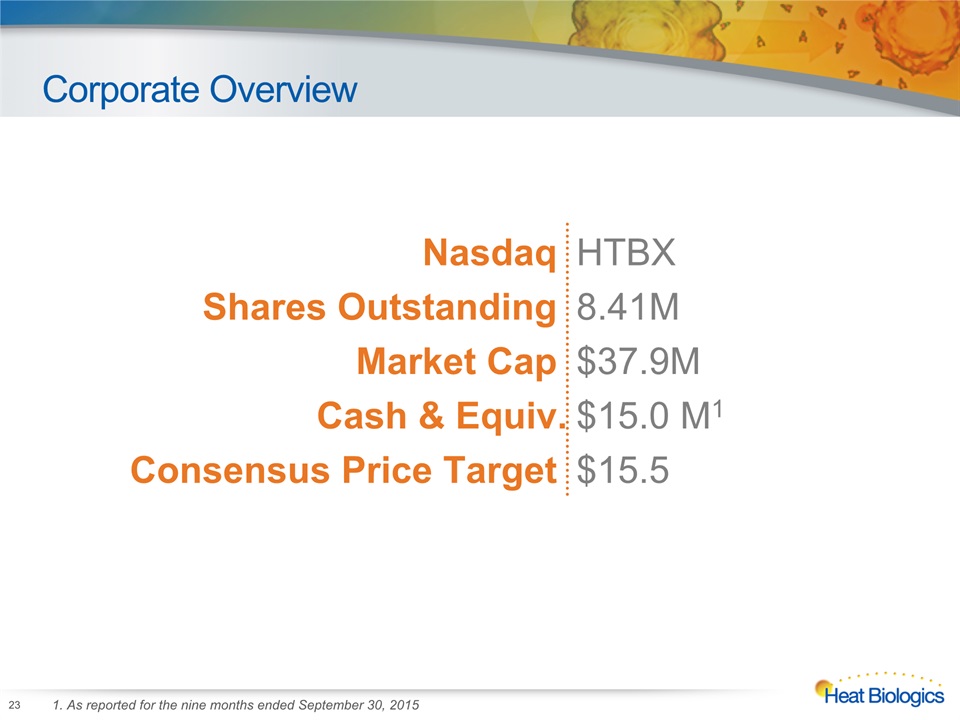
Corporate Overview 23 1. As reported for the nine months ended September 30, 2015 Nasdaq HTBX Shares Outstanding 8.41M Market Cap $37.9M Cash & Equiv. $15.0 M1 Consensus Price Target $15.5

APPENDIX
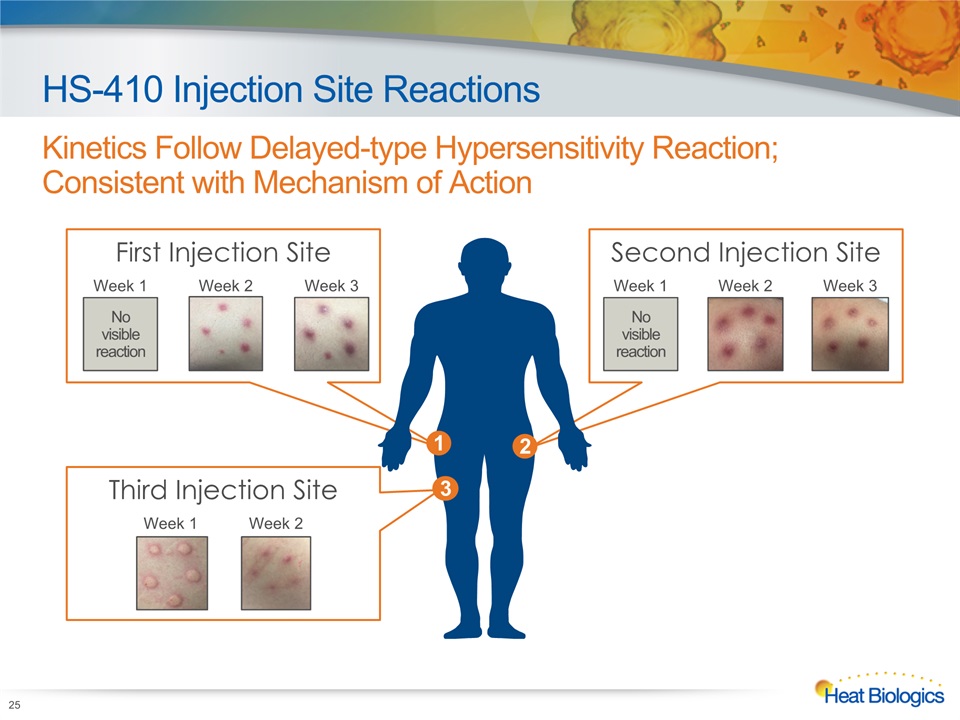
Third Injection Site First Injection Site Second Injection Site HS-410 Injection Site Reactions Kinetics Follow Delayed-type Hypersensitivity Reaction; Consistent with Mechanism of Action 25 No visible reaction Week 1 Week 2 Week 3 No visible reaction 1 2 3 Week 1 Week 2 Week 3 Week 1 Week 2
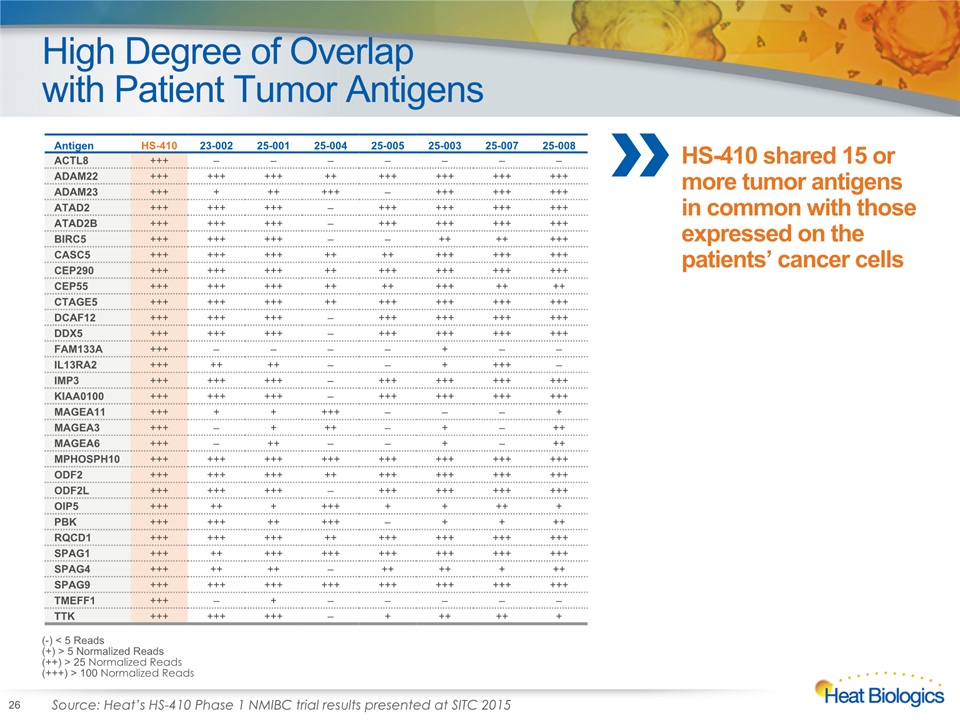
High Degree of Overlapwith Patient Tumor Antigens 26 Recurrent DiseaseDisease-free (-) <5 Reads(+) >5 Normalized Reads(++) >25 Normalized Reads(+++) >100 Normalized Reads C Antigen HS-410 23-002 25-001 25-004 25-005 25-003 25-007 25-008 ACTL8 +++ – – – – – – – ADAM22 +++ +++ +++ ++ +++ +++ +++ +++ ADAM23 +++ + ++ +++ – +++ +++ +++ ATAD2 +++ +++ +++ – +++ +++ +++ +++ ATAD2B +++ +++ +++ – +++ +++ +++ +++ BIRC5 +++ +++ +++ – – ++ ++ +++ CASC5 +++ +++ +++ ++ ++ +++ +++ +++ CEP290 +++ +++ +++ ++ +++ +++ +++ +++ CEP55 +++ +++ +++ ++ ++ +++ ++ ++ CTAGE5 +++ +++ +++ ++ +++ +++ +++ +++ DCAF12 +++ +++ +++ – +++ +++ +++ +++ DDX5 +++ +++ +++ – +++ +++ +++ +++ FAM133A +++ – – – – + – – IL13RA2 +++ ++ ++ – – + +++ – IMP3 +++ +++ +++ – +++ +++ +++ +++ KIAA0100 +++ +++ +++ – +++ +++ +++ +++ MAGEA11 +++ + + +++ – – – + MAGEA3 +++ – + ++ – + – ++ MAGEA6 +++ – ++ – – + – ++ MPHOSPH10 +++ +++ +++ +++ +++ +++ +++ +++ ODF2 +++ +++ +++ ++ +++ +++ +++ +++ ODF2L +++ +++ +++ – +++ +++ +++ +++ OIP5 +++ ++ + +++ + + ++ + PBK +++ +++ ++ +++ – + + ++ RQCD1 +++ +++ +++ ++ +++ +++ +++ +++ SPAG1 +++ ++ +++ +++ +++ +++ +++ +++ SPAG4 +++ ++ ++ – ++ ++ + ++ SPAG9 +++ +++ +++ +++ +++ +++ +++ +++ TMEFF1 +++ – + – – – – – TTK +++ +++ +++ – + ++ ++ + (-) < 5 Reads(+) > 5 Normalized Reads(++) > 25 Normalized Reads(+++) > 100 Normalized Reads HS-410 shared 15 or more tumor antigens in common with those expressed on the patients’ cancer cells Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015

THANK YOU