POWER POINT PRESENTATION
Published on June 6, 2016
EXHIBIT 99.1

NASDAQ:HTBX June 2016

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2015 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2
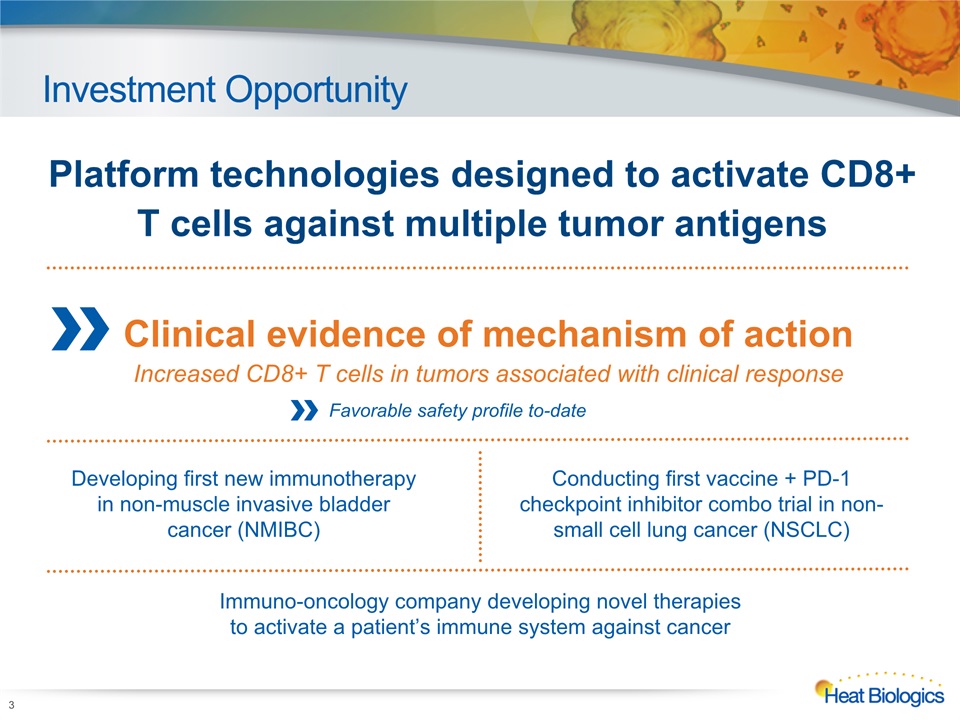
Investment Opportunity 3 Developing first new immunotherapy in non-muscle invasive bladder cancer (NMIBC) Platform technologies designed to activate CD8+ T cells against multiple tumor antigens Immuno-oncology company developing novel therapies to activate a patient’s immune system against cancer Conducting first vaccine + PD-1 checkpoint inhibitor combo trial in non-small cell lung cancer (NSCLC) Clinical evidence of mechanism of actionIncreased CD8+ T cells in tumors associated with clinical response Favorable safety profile to-date
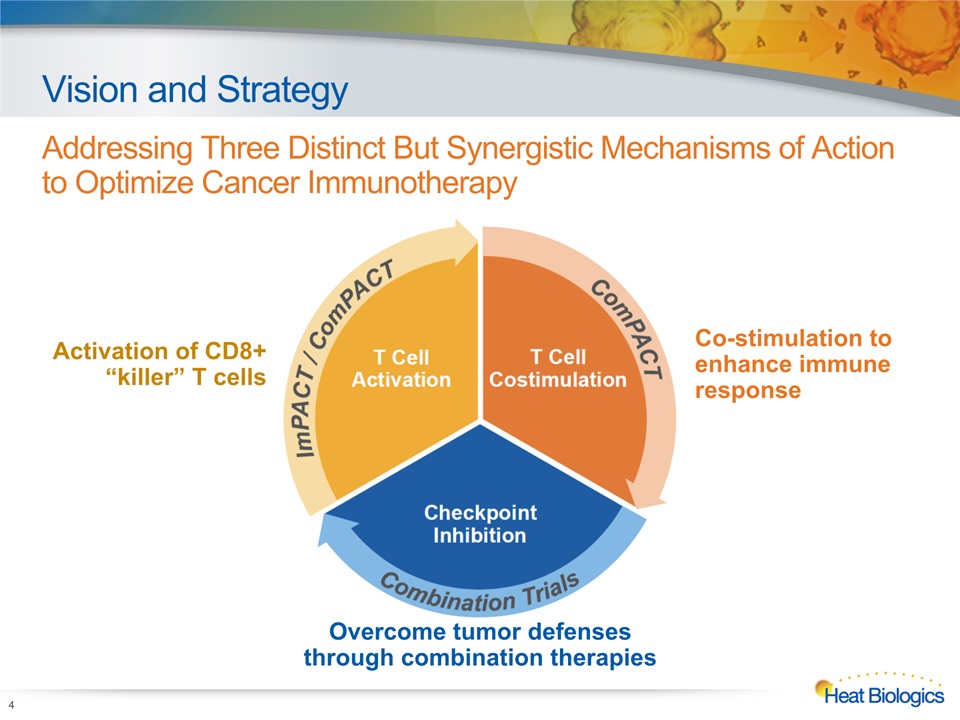
Vision and Strategy Addressing Three Distinct But Synergistic Mechanisms of Action to Optimize Cancer Immunotherapy 4 Activation of CD8+ “killer” T cells Co-stimulation to enhance immune response Overcome tumor defenses through combination therapies
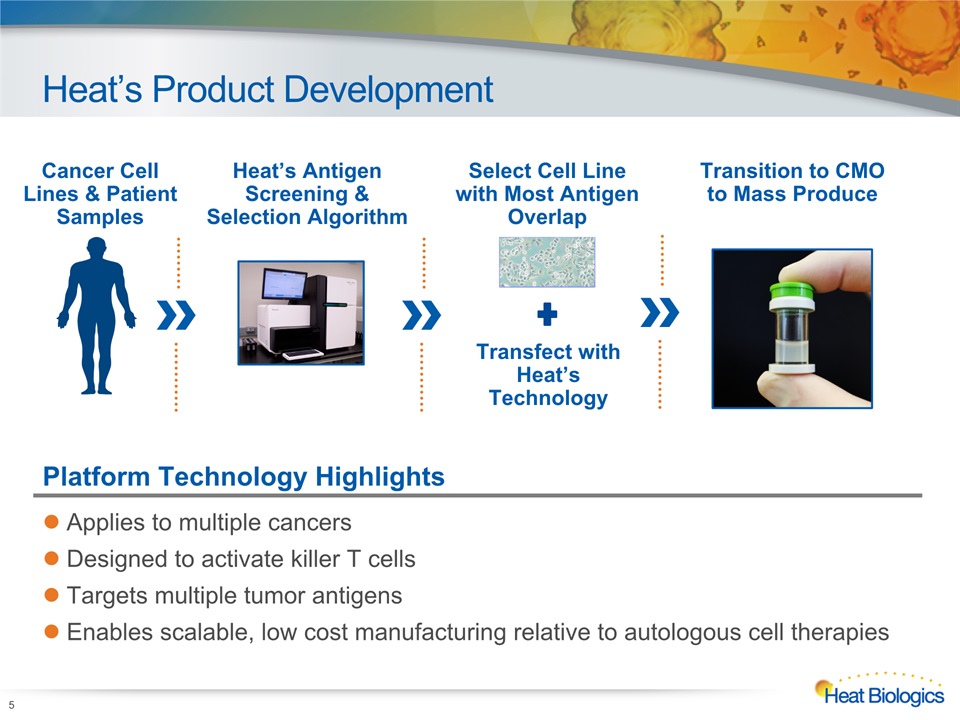
Heat’s Product Development 5 Cancer Cell Lines & Patient Samples Heat’s AntigenScreening & Selection Algorithm Transition to CMOto Mass Produce Select Cell Line with Most Antigen Overlap Transfect with Heat’s Technology Platform Technology Highlights Applies to multiple cancersDesigned to activate killer T cellsTargets multiple tumor antigensEnables scalable, low cost manufacturing relative to autologous cell therapies
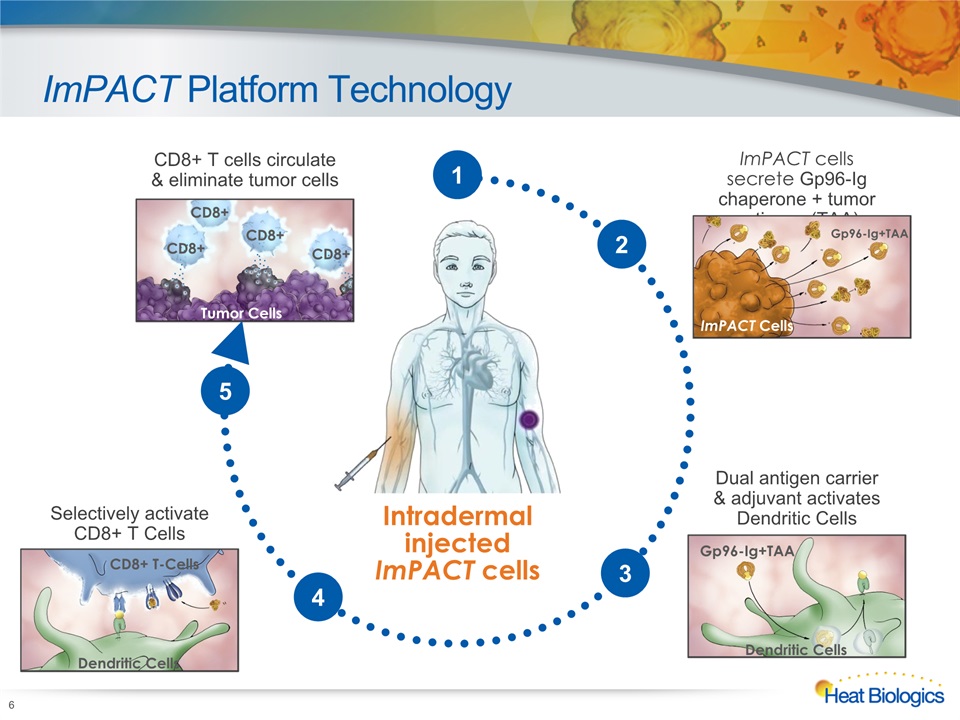
ImPACT Platform Technology 6 Intradermal injectedImPACT cells ImPACT cells secrete Gp96-Ig chaperone + tumor antigens (TAA) ImPACT Cells Gp96-Ig+TAA Selectively activate CD8+ T Cells CD8+ T-Cells Dendritic Cells CD8+ T cells circulate & eliminate tumor cells Tumor Cells CD8+ CD8+ CD8+ CD8+ 2 Dual antigen carrier & adjuvant activates Dendritic Cells Gp96-Ig+TAA Dendritic Cells 3 4 5 1
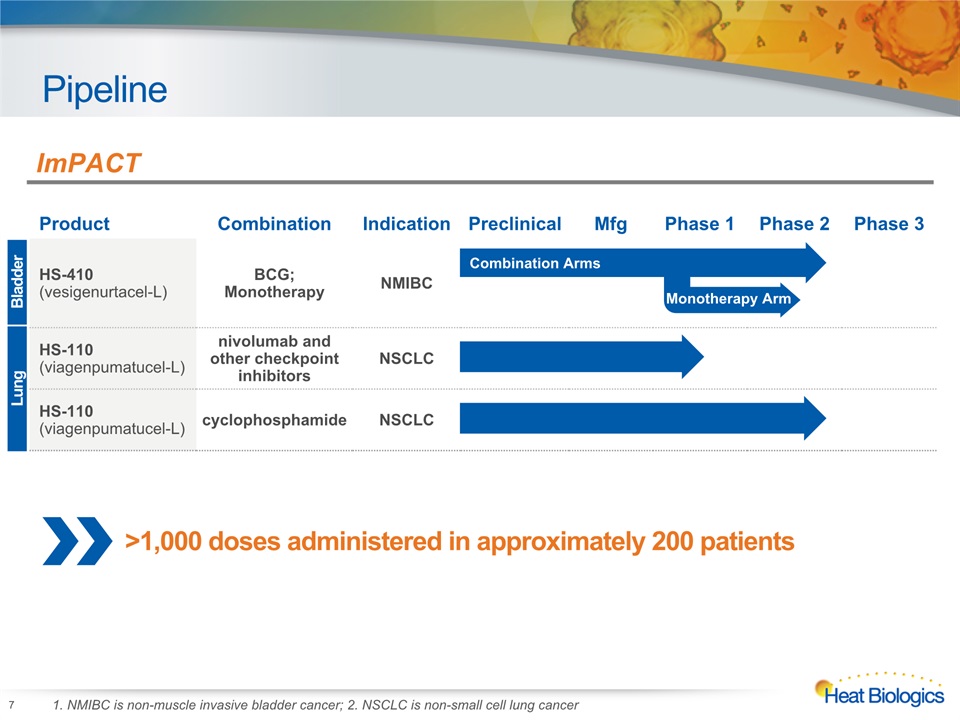
Pipeline 7 Heat Biologics Corporate Model Deck Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) BCG; Monotherapy NMIBC HS-110(viagenpumatucel-L) nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC Bladder Lung ImPACT Monotherapy Arm Combination Arms 1. NMIBC is non-muscle invasive bladder cancer; 2. NSCLC is non-small cell lung cancer >1,000 doses administered in approximately 200 patients
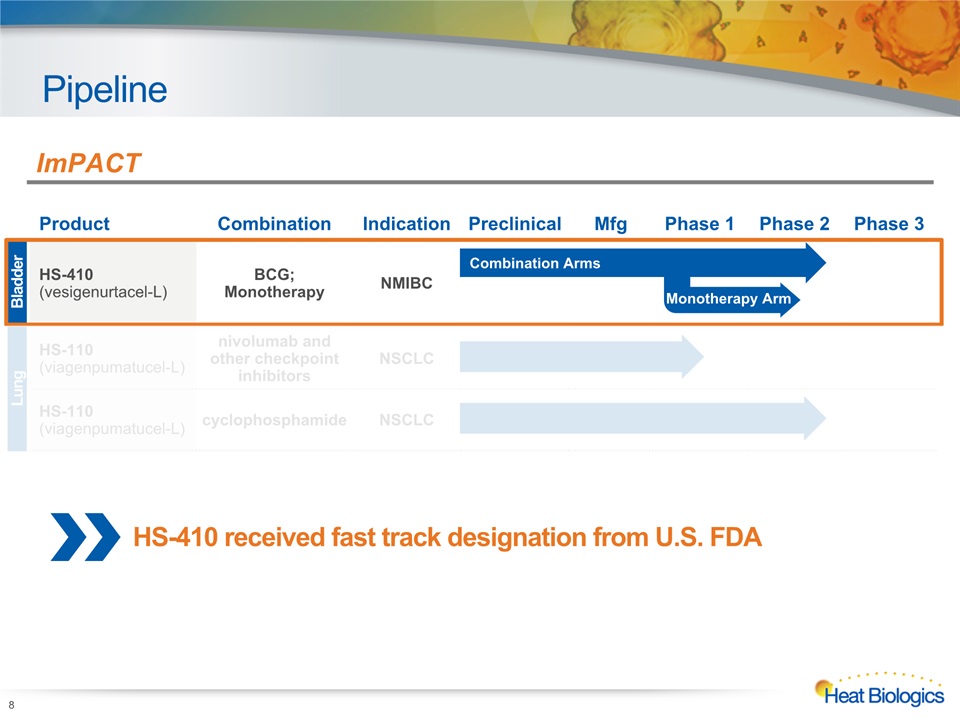
Pipeline 8 Heat Biologics Corporate Model Deck HS-410 received fast track designation from U.S. FDA Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) BCG;Monotherapy NMIBC HS-110(viagenpumatucel-L) nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC Bladder Lung ImPACT Combination Arms Monotherapy Arm
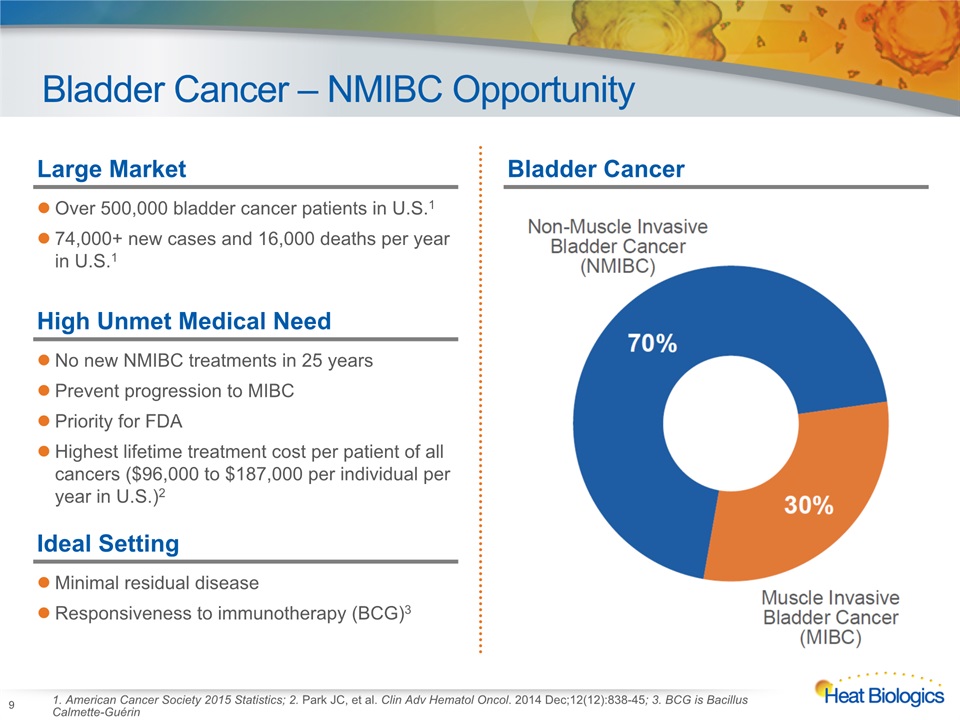
Bladder Cancer – NMIBC Opportunity 9 1. American Cancer Society 2015 Statistics; 2. Park JC, et al. Clin Adv Hematol Oncol. 2014 Dec;12(12):838-45; 3. BCG is Bacillus Calmette-Guérin Large MarketOver 500,000 bladder cancer patients in U.S.174,000+ new cases and 16,000 deaths per year in U.S.1 High Unmet Medical NeedNo new NMIBC treatments in 25 yearsPrevent progression to MIBCPriority for FDAHighest lifetime treatment cost per patient of all cancers ($96,000 to $187,000 per individual per year in U.S.)2 Ideal SettingMinimal residual diseaseResponsiveness to immunotherapy (BCG)3 Bladder Cancer
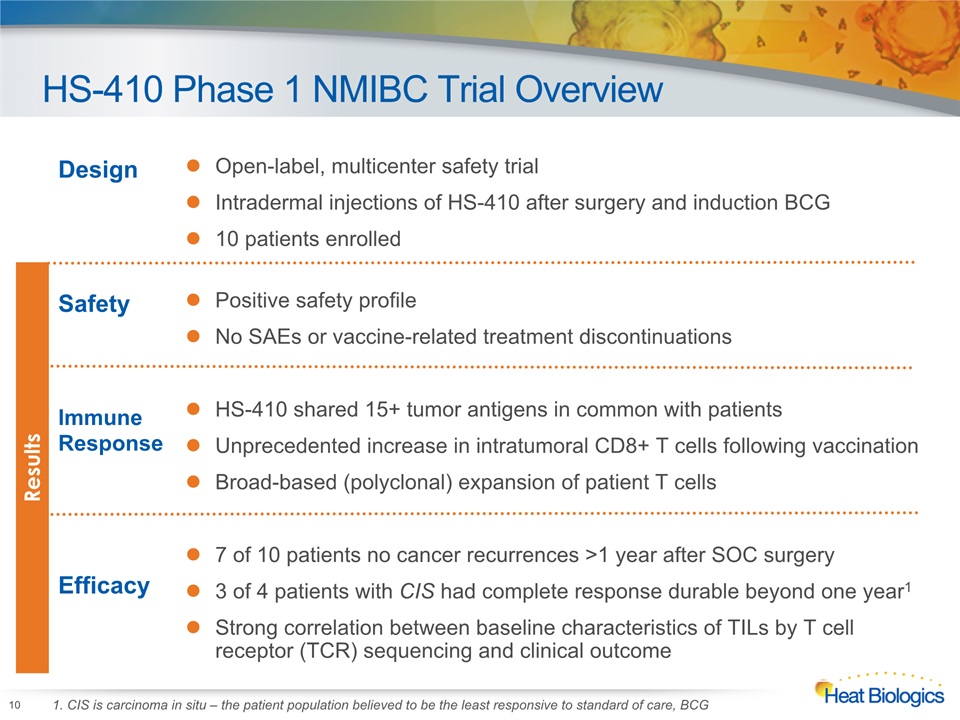
HS-410 Phase 1 NMIBC Trial Overview Design Open-label, multicenter safety trialIntradermal injections of HS-410 after surgery and induction BCG10 patients enrolled SafetyImmune ResponseEfficacy Positive safety profileNo SAEs or vaccine-related treatment discontinuationsHS-410 shared 15+ tumor antigens in common with patientsUnprecedented increase in intratumoral CD8+ T cells following vaccination Broad-based (polyclonal) expansion of patient T cells7 of 10 patients no cancer recurrences >1 year after SOC surgery3 of 4 patients with CIS had complete response durable beyond one year1Strong correlation between baseline characteristics of TILs by T cell receptor (TCR) sequencing and clinical outcome 10 Results 1. CIS is carcinoma in situ – the patient population believed to be the least responsive to standard of care, BCG
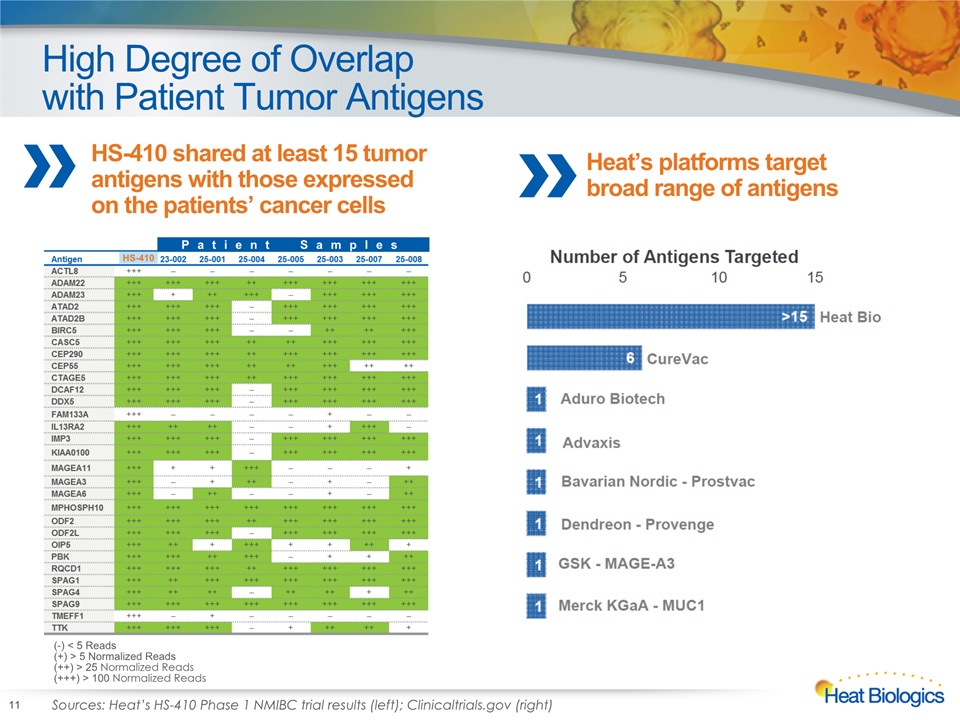
High Degree of Overlapwith Patient Tumor Antigens 11 (-) < 5 Reads(+) > 5 Normalized Reads(++) > 25 Normalized Reads(+++) > 100 Normalized Reads HS-410 shared at least 15 tumor antigens with those expressed on the patients’ cancer cells Heat’s platforms target broad range of antigens Patient Samples HS-410 Recurrent DiseaseDisease-free (-) <5 Reads(+) >5 Normalized Reads(++) >25 Normalized Reads(+++) >100 Normalized Reads C Sources: Heat’s HS-410 Phase 1 NMIBC trial results (left); Clinicaltrials.gov (right)
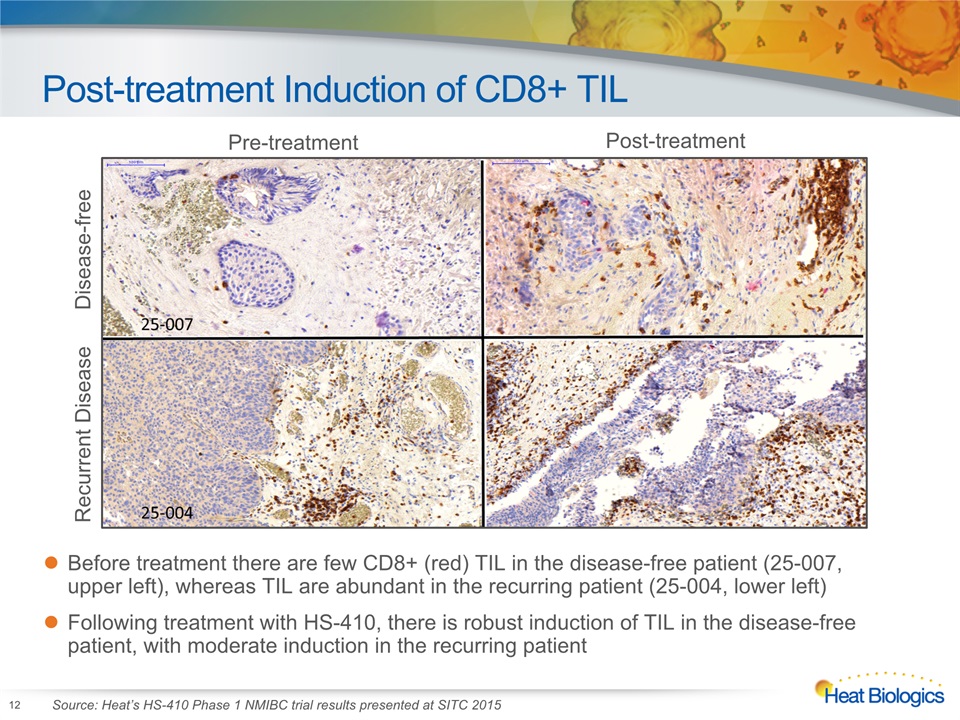
Post-treatment Induction of CD8+ TIL 12 Before treatment there are few CD8+ (red) TIL in the disease-free patient (25-007, upper left), whereas TIL are abundant in the recurring patient (25-004, lower left)Following treatment with HS-410, there is robust induction of TIL in the disease-free patient, with moderate induction in the recurring patient Pre-treatment Post-treatment Recurrent Disease Disease-free Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015
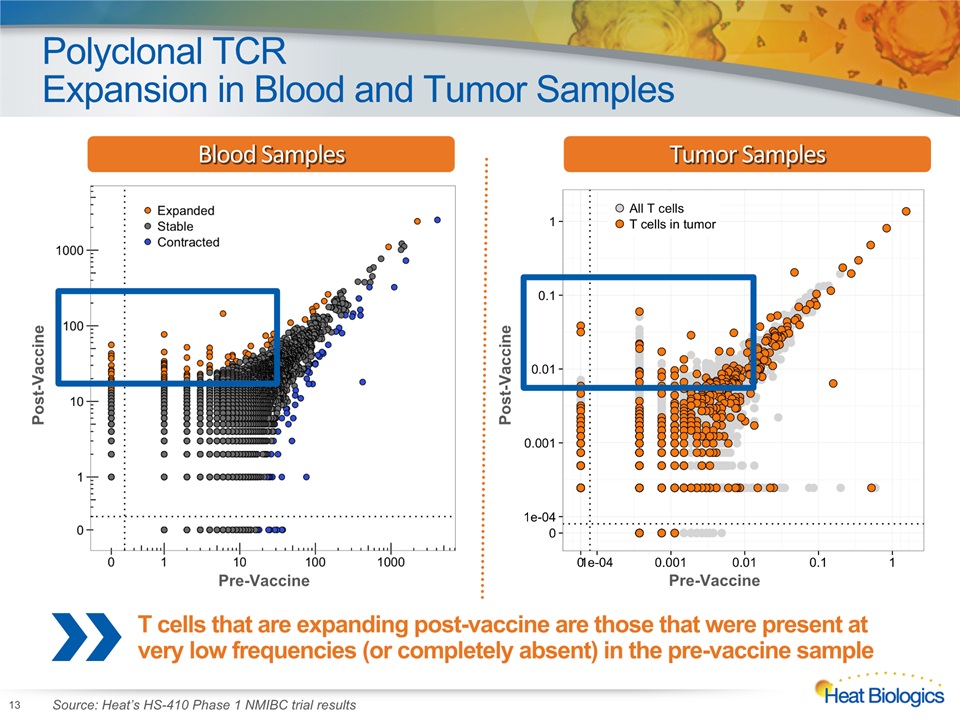
Polyclonal TCRExpansion in Blood and Tumor Samples 13 Source: Heat’s HS-410 Phase 1 NMIBC trial results T cells that are expanding post-vaccine are those that were present at very low frequencies (or completely absent) in the pre-vaccine sample Blood Samples Post-Vaccine Post-Vaccine Pre-Vaccine Pre-Vaccine Tumor Samples
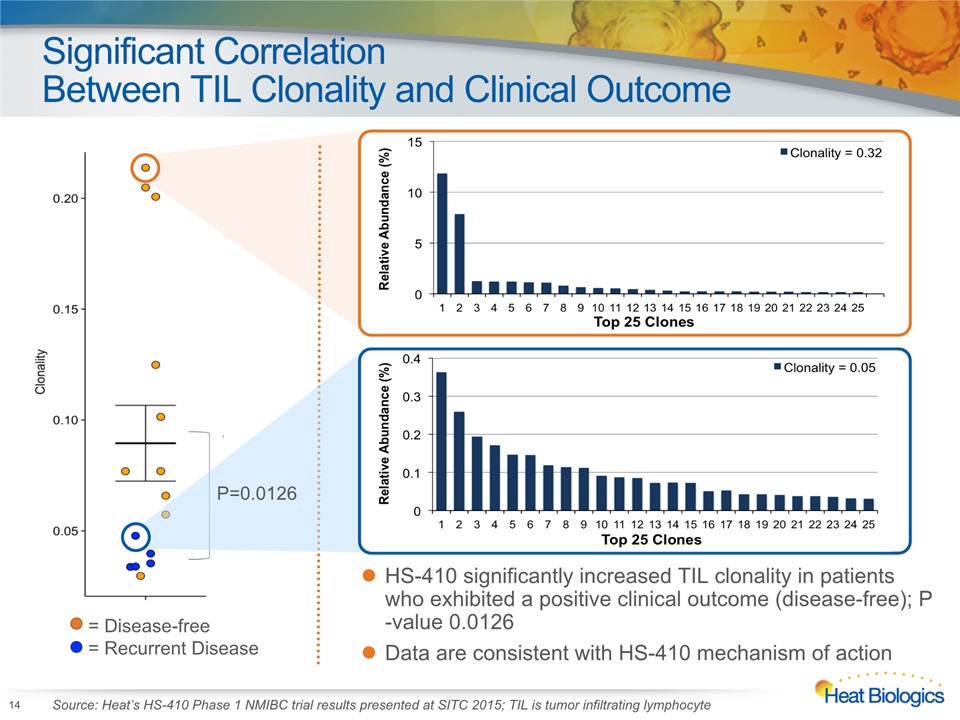
Significant CorrelationBetween TIL Clonality and Clinical Outcome 14 HS-410 significantly increased TIL clonality in patientswho exhibited a positive clinical outcome (disease-free); P-value 0.0126 Data are consistent with HS-410 mechanism of action = Disease-free = Recurrent Disease Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015; TIL is tumor infiltrating lymphocyte P=0.0126
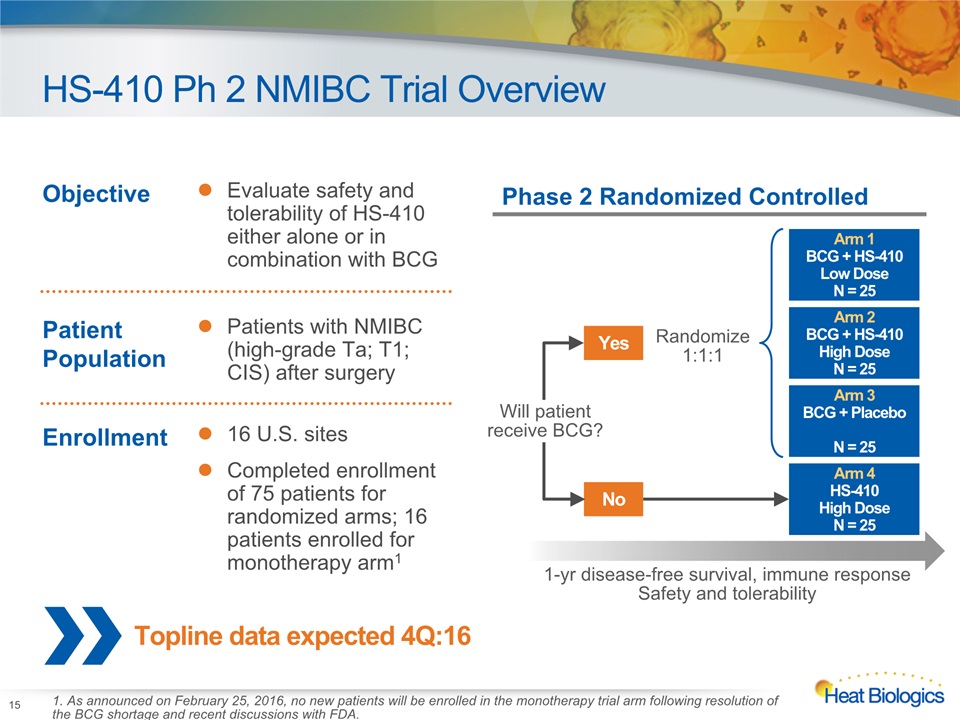
HS-410 Ph 2 NMIBC Trial Overview 15 Topline data expected 4Q:16 Objective Evaluate safety and tolerability of HS-410 either alone or in combination with BCG Patient Population Patients with NMIBC (high-grade Ta; T1; CIS) after surgery Enrollment 16 U.S. sitesCompleted enrollment of 75 patients for randomized arms; 16 patients enrolled for monotherapy arm1 Phase 2 Randomized Controlled Arm 2BCG + HS-410High DoseN = 25 Arm 1BCG + HS-410Low DoseN = 25 Arm 3BCG + PlaceboN = 25 Arm 4HS-410 High DoseN = 25 Yes Randomize1:1:1 No Will patientreceive BCG? 1-yr disease-free survival, immune responseSafety and tolerability 1. As announced on February 25, 2016, no new patients will be enrolled in the monotherapy trial arm following resolution of the BCG shortage and recent discussions with FDA.
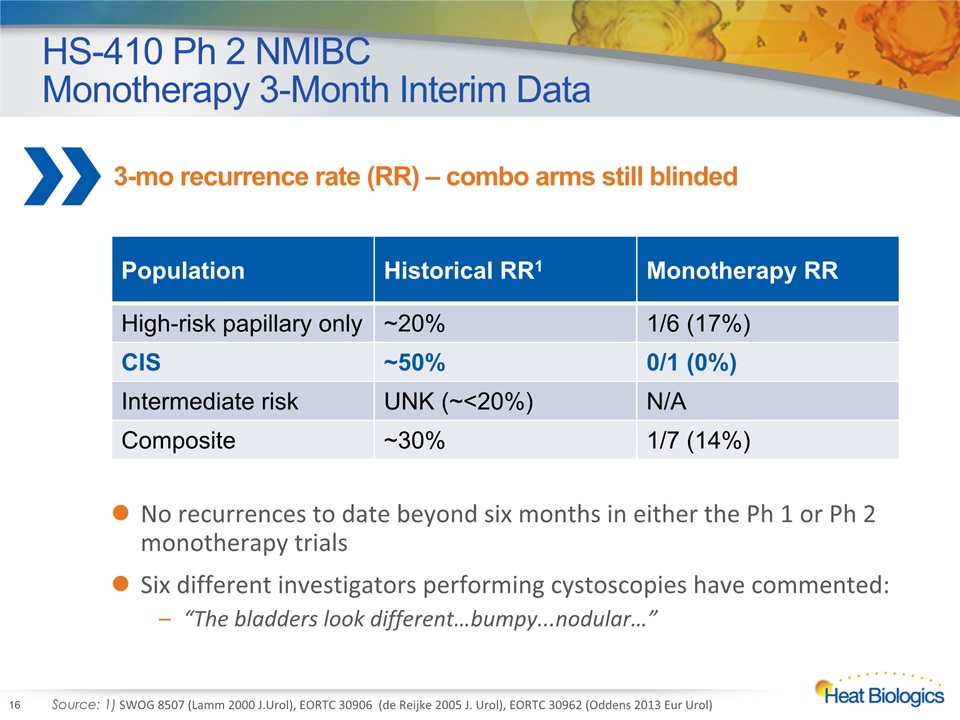
HS-410 Ph 2 NMIBC Monotherapy 3-Month Interim Data 16 Population Historical RR1 Monotherapy RR High-risk papillary only ~20% 1/6 (17%) CIS ~50% 0/1 (0%) Intermediate risk UNK (~<20%) N/A Composite ~30% 1/7 (14%) Source: 1) SWOG 8507 (Lamm 2000 J.Urol), EORTC 30906 (de Reijke 2005 J. Urol), EORTC 30962 (Oddens 2013 Eur Urol) No recurrences to date beyond six months in either the Ph 1 or Ph 2 monotherapy trialsSix different investigators performing cystoscopies have commented:“The bladders look different…bumpy...nodular…” 3-mo recurrence rate (RR) – combo arms still blinded
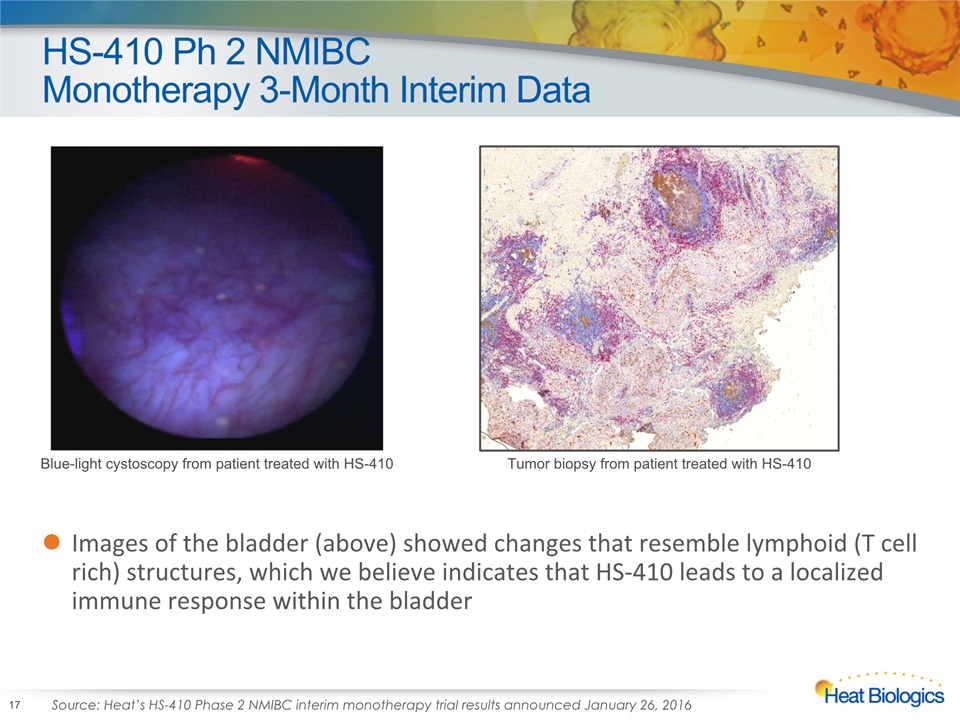
HS-410 Ph 2 NMIBC Monotherapy 3-Month Interim Data Images of the bladder (above) showed changes that resemble lymphoid (T cell rich) structures, which we believe indicates that HS-410 leads to a localized immune response within the bladder Blue-light cystoscopy from patient treated with HS-410 Tumor biopsy from patient treated with HS-410 Source: Heat’s HS-410 Phase 2 NMIBC interim monotherapy trial results announced January 26, 2016 17

Pipeline 18 Heat Biologics Corporate Model Deck Product Combination Indication Preclinical Mfg Phase 1 Phase 2 Phase 3 HS-410(vesigenurtacel-L) BCG;Monotherapy NMIBC HS-110(viagenpumatucel-L) nivolumab and other checkpoint inhibitors NSCLC HS-110 (viagenpumatucel-L) cyclophosphamide NSCLC Bladder Lung ImPACT Combination Arms Monotherapy Arm
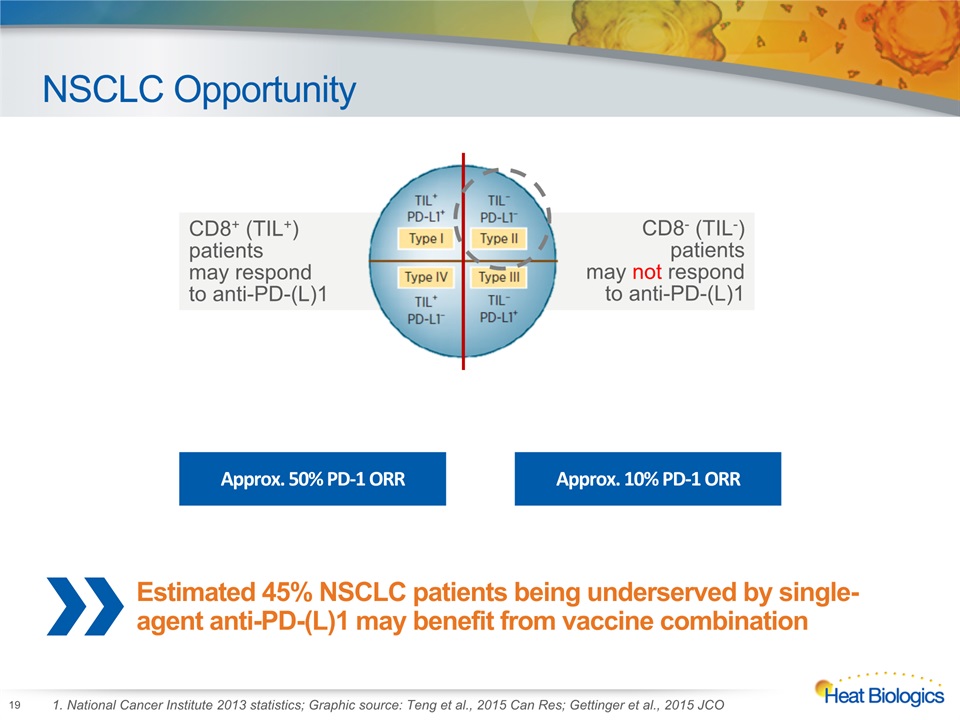
NSCLC Opportunity 19 1. National Cancer Institute 2013 statistics; Graphic source: Teng et al., 2015 Can Res; Gettinger et al., 2015 JCO CD8+ (TIL+) patients may respond to anti-PD-(L)1 CD8- (TIL-) patients may not respond to anti-PD-(L)1 Approx. 50% PD-1 ORR Approx. 10% PD-1 ORR Estimated 45% NSCLC patients being underserved by single-agent anti-PD-(L)1 may benefit from vaccine combination
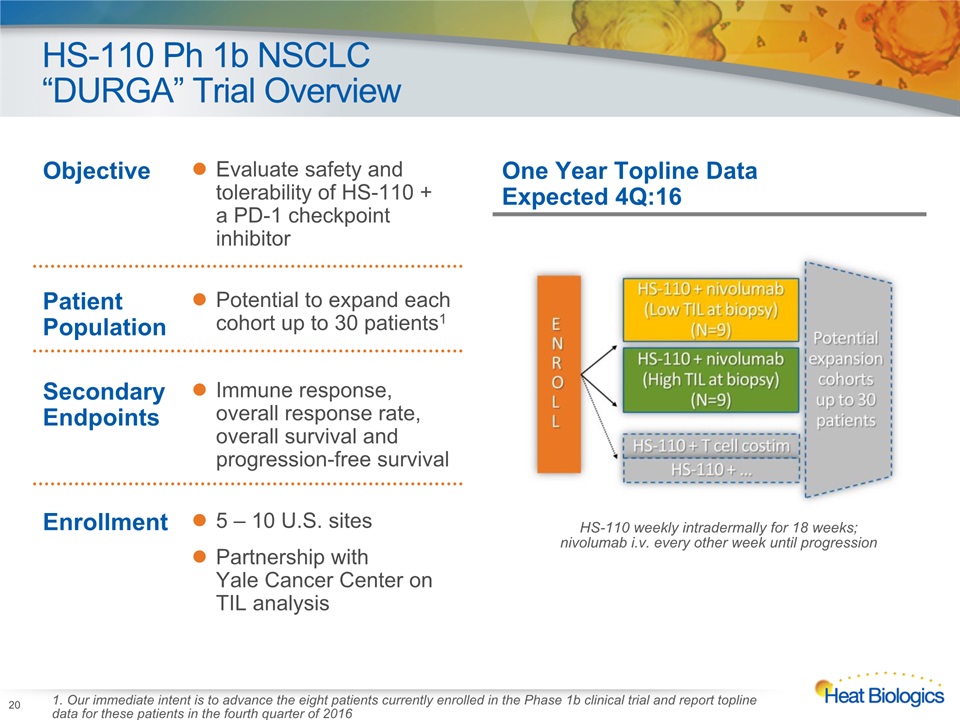
HS-110 Ph 1b NSCLC “DURGA” Trial Overview HS-110 weekly intradermally for 18 weeks; nivolumab i.v. every other week until progression One Year Topline Data Expected 4Q:16 Objective Evaluate safety and tolerability of HS-110 + a PD-1 checkpoint inhibitor Patient Population Potential to expand each cohort up to 30 patients1 Secondary Endpoints Immune response, overall response rate, overall survival and progression-free survival Enrollment 5 – 10 U.S. sitesPartnership with Yale Cancer Center on TIL analysis 20 1. Our immediate intent is to advance the eight patients currently enrolled in the Phase 1b clinical trial and report topline data for these patients in the fourth quarter of 2016
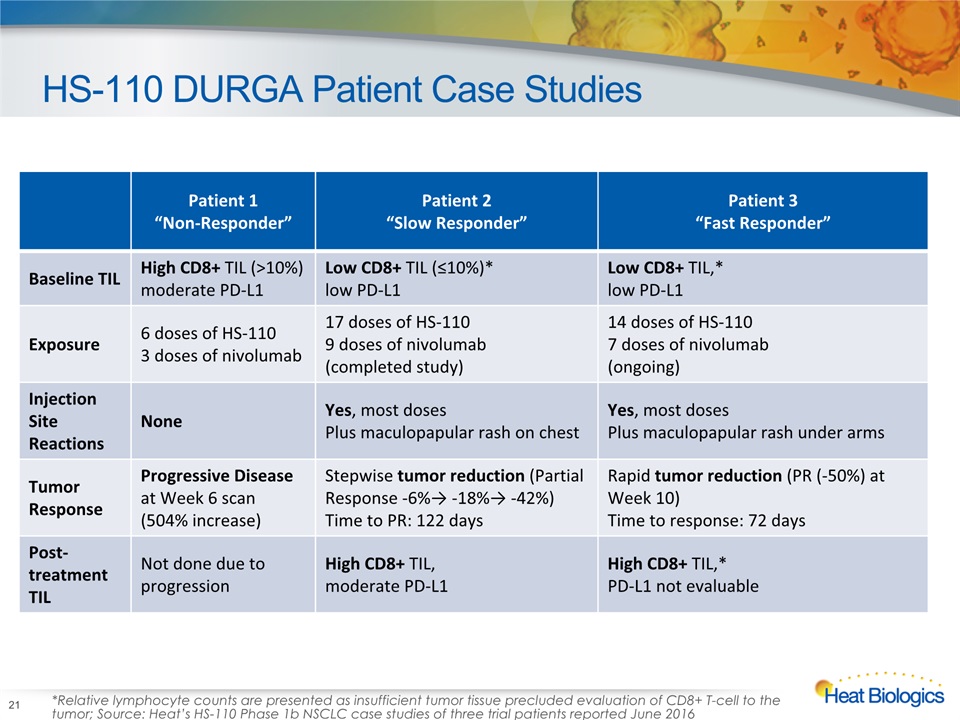
HS-110 DURGA Patient Case Studies 21 Patient 1 “Non-Responder” Patient 2 “Slow Responder” Patient 3 “Fast Responder” Baseline TIL High CD8+ TIL (>10%)moderate PD-L1 Low CD8+ TIL (≤10%)*low PD-L1 Low CD8+ TIL,* low PD-L1 Exposure 6 doses of HS-110 3 doses of nivolumab 17 doses of HS-110 9 doses of nivolumab (completed study) 14 doses of HS-1107 doses of nivolumab(ongoing) Injection Site Reactions None Yes, most dosesPlus maculopapular rash on chest Yes, most dosesPlus maculopapular rash under arms Tumor Response Progressive Disease at Week 6 scan (504% increase) Stepwise tumor reduction (Partial Response -6%→ -18%→ -42%)Time to PR: 122 days Rapid tumor reduction (PR (-50%) at Week 10)Time to response: 72 days Post-treatment TIL Not done due to progression High CD8+ TIL, moderate PD-L1 High CD8+ TIL,* PD-L1 not evaluable *Relative lymphocyte counts are presented as insufficient tumor tissue precluded evaluation of CD8+ T-cell to the tumor; Source: Heat’s HS-110 Phase 1b NSCLC case studies of three trial patients reported June 2016
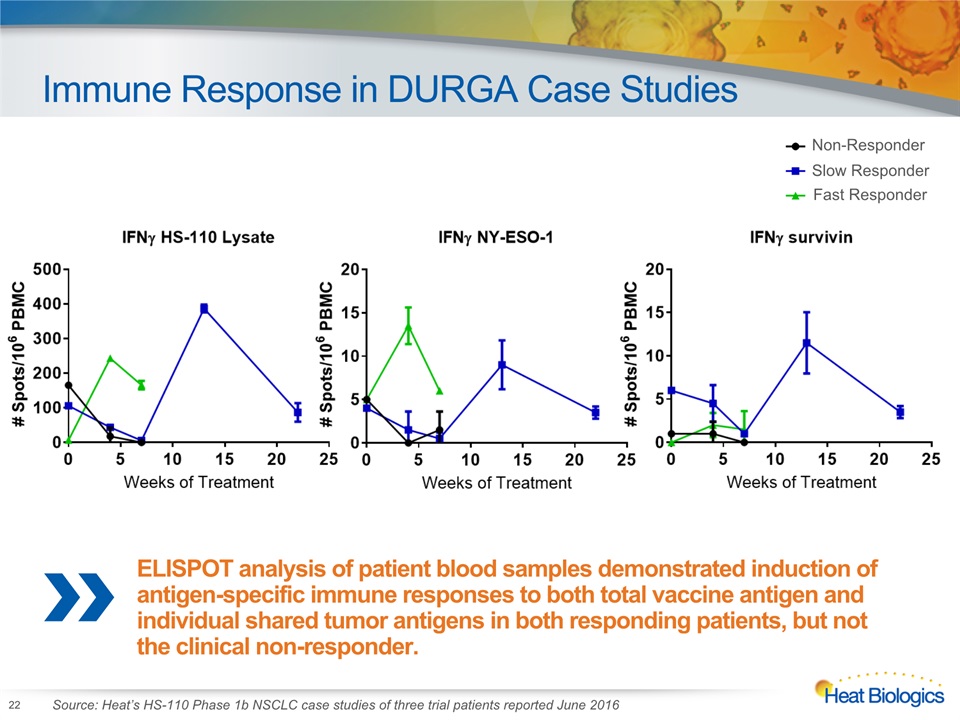
Immune Response in DURGA Case Studies 22 Fast Responder Slow Responder Non-Responder ELISPOT analysis of patient blood samples demonstrated induction of antigen-specific immune responses to both total vaccine antigen and individual shared tumor antigens in both responding patients, but not the clinical non-responder. Source: Heat’s HS-110 Phase 1b NSCLC case studies of three trial patients reported June 2016

ComPACT Platform Technology 23 gp96-Ig OX40L-Fc The first potential dual-acting immunotherapy designed to deliver T cell activation and costimulation in a single product – combination therapy without additive costs
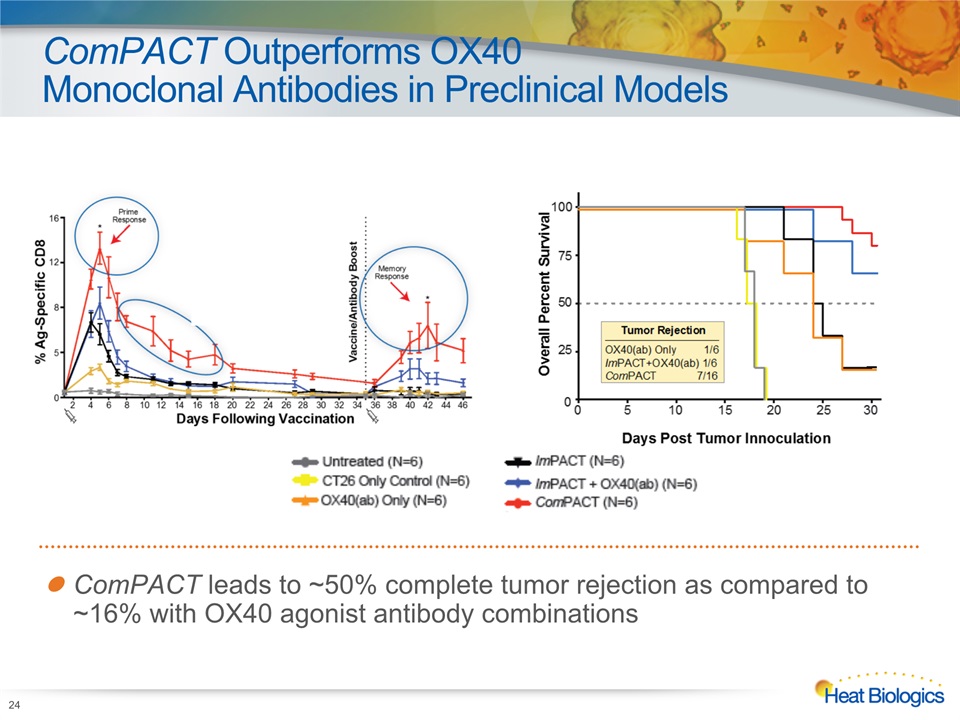
ComPACT Outperforms OX40 Monoclonal Antibodies in Preclinical Models 24 ComPACT leads to ~50% complete tumor rejection as compared to ~16% with OX40 agonist antibody combinations
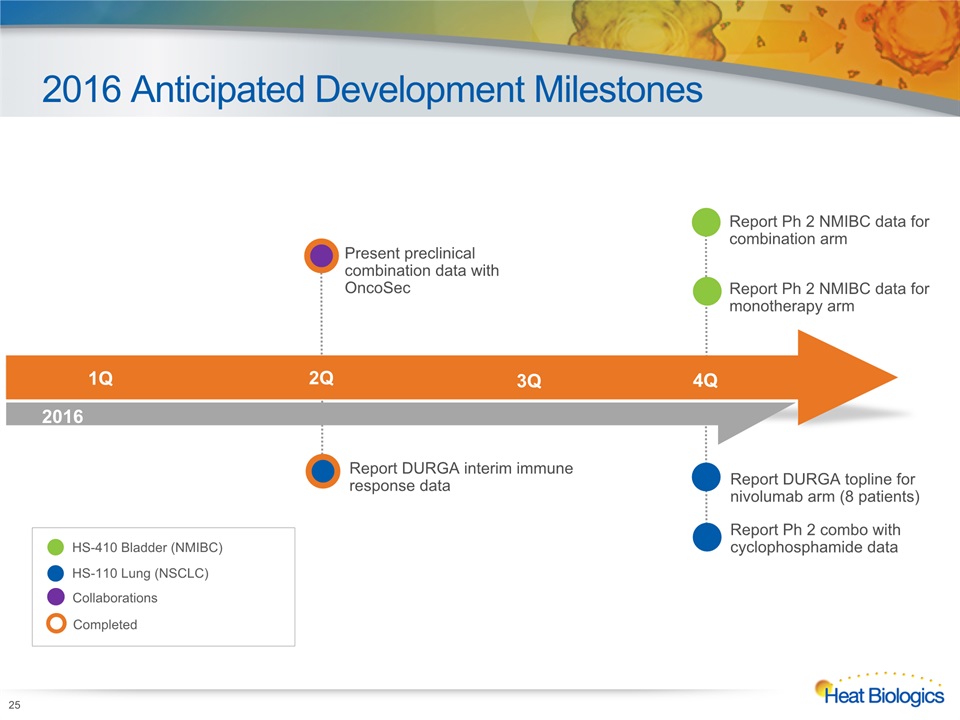
2016 Anticipated Development Milestones 25 Report DURGA interim immune response data HS-410 Bladder (NMIBC) HS-110 Lung (NSCLC) 1Q 2Q 3Q 4Q 2015 2016 Completed Report DURGA topline for nivolumab arm (8 patients)Report Ph 2 combo with cyclophosphamide data Report Ph 2 NMIBC data for combination armReport Ph 2 NMIBC data for monotherapy arm Collaborations Present preclinical combination data with OncoSec
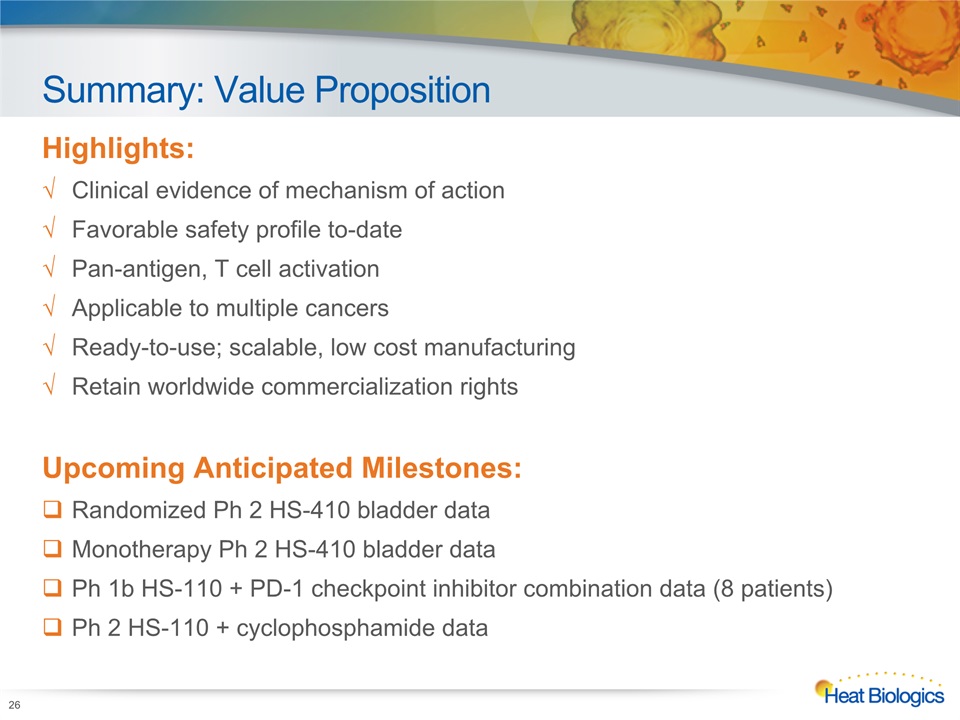
Summary: Value Proposition Highlights:Clinical evidence of mechanism of actionFavorable safety profile to-datePan-antigen, T cell activationApplicable to multiple cancersReady-to-use; scalable, low cost manufacturingRetain worldwide commercialization rightsUpcoming Anticipated Milestones:Randomized Ph 2 HS-410 bladder dataMonotherapy Ph 2 HS-410 bladder dataPh 1b HS-110 + PD-1 checkpoint inhibitor combination data (8 patients) Ph 2 HS-110 + cyclophosphamide data 26

APPENDIX
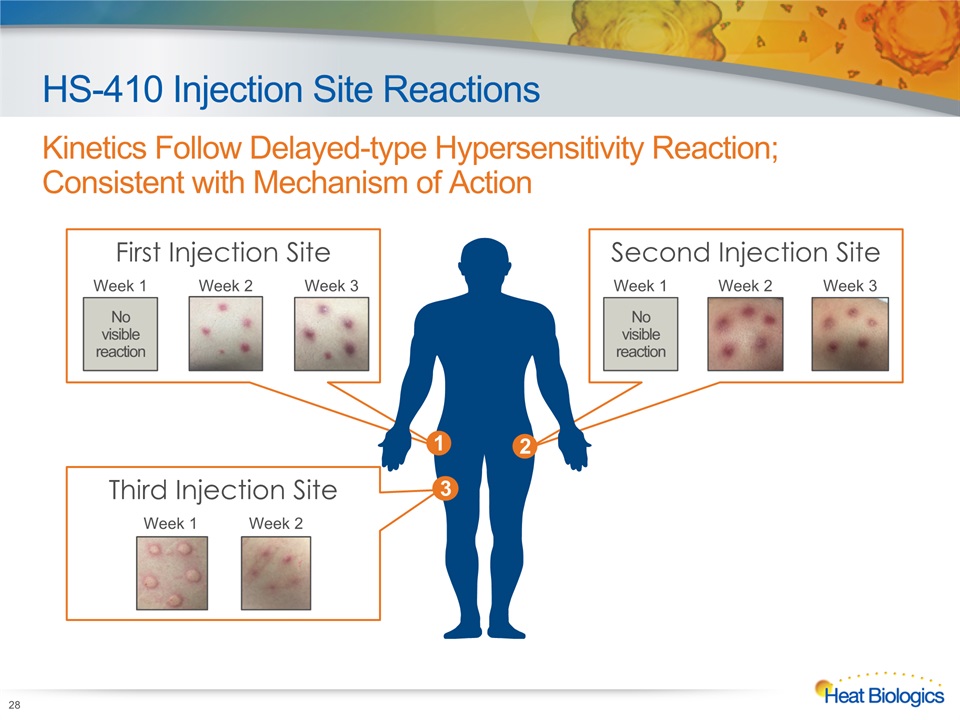
Third Injection Site First Injection Site Second Injection Site HS-410 Injection Site Reactions Kinetics Follow Delayed-type Hypersensitivity Reaction; Consistent with Mechanism of Action 28 No visible reaction Week 1 Week 2 Week 3 No visible reaction 1 2 3 Week 1 Week 2 Week 3 Week 1 Week 2
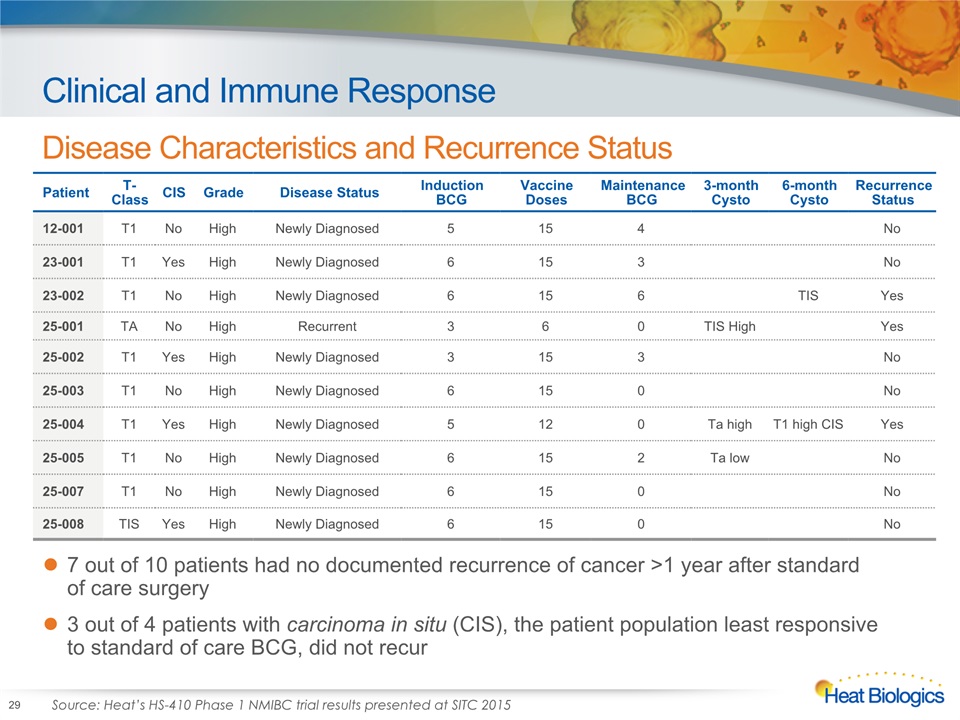
Clinical and Immune Response Disease Characteristics and Recurrence Status 29 Patient T-Class CIS Grade Disease Status Induction BCG Vaccine Doses Maintenance BCG 3-month Cysto 6-month Cysto Recurrence Status 12-001 T1 No High Newly Diagnosed 5 15 4 No 23-001 T1 Yes High Newly Diagnosed 6 15 3 No 23-002 T1 No High Newly Diagnosed 6 15 6 TIS Yes 25-001 TA No High Recurrent 3 6 0 TIS High Yes 25-002 T1 Yes High Newly Diagnosed 3 15 3 No 25-003 T1 No High Newly Diagnosed 6 15 0 No 25-004 T1 Yes High Newly Diagnosed 5 12 0 Ta high T1 high CIS Yes 25-005 T1 No High Newly Diagnosed 6 15 2 Ta low No 25-007 T1 No High Newly Diagnosed 6 15 0 No 25-008 TIS Yes High Newly Diagnosed 6 15 0 No 7 out of 10 patients had no documented recurrence of cancer >1 year after standard of care surgery3 out of 4 patients with carcinoma in situ (CIS), the patient population least responsive to standard of care BCG, did not recur Source: Heat’s HS-410 Phase 1 NMIBC trial results presented at SITC 2015
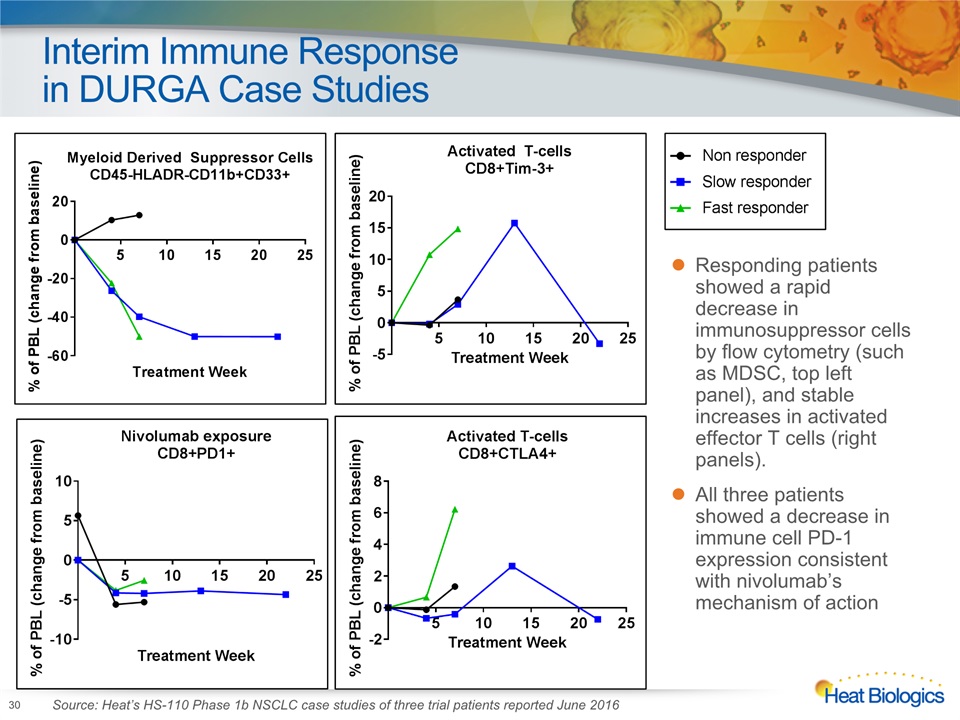
Interim Immune Response in DURGA Case Studies 30 Responding patients showed a rapid decrease in immunosuppressor cells by flow cytometry (such as MDSC, top left panel), and stable increases in activated effector T cells (right panels). All three patients showed a decrease in immune cell PD-1 expression consistent with nivolumab’s mechanism of action Source: Heat’s HS-110 Phase 1b NSCLC case studies of three trial patients reported June 2016

THANK YOU