POWER POINT PRESENTATION
Published on February 28, 2018
EXHIBIT 99.2
1 Heat Biologics Analyst and Investor DayFebruary 28, 2018

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, future trial results being consistent with interim results, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our most recent Annual Report on Form 10-K and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business.

Roger B. Cohen Professor of MedicineUniversity of PennsylvaniaAssociate Director Clinical Research Abramson Cancer CenterChief Clinical Research Officer Abramson Cancer CenterCo-Director, Head and Neck Cancer Research Center
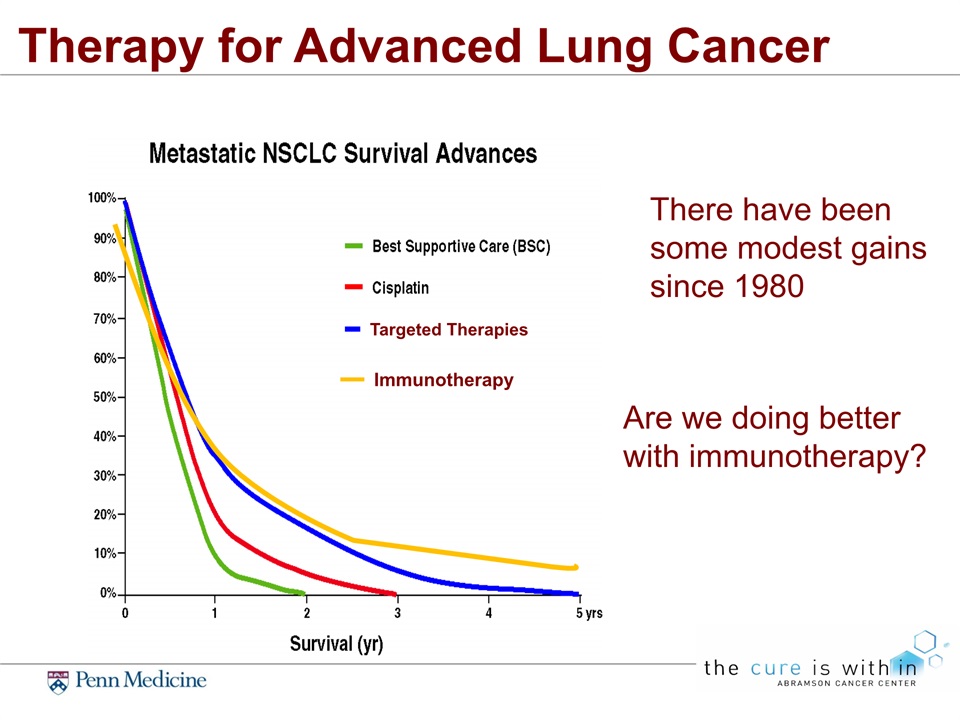
Therapy for Advanced Lung Cancer There have been some modest gains since 1980 Immunotherapy Are we doing better with immunotherapy? Targeted Therapies
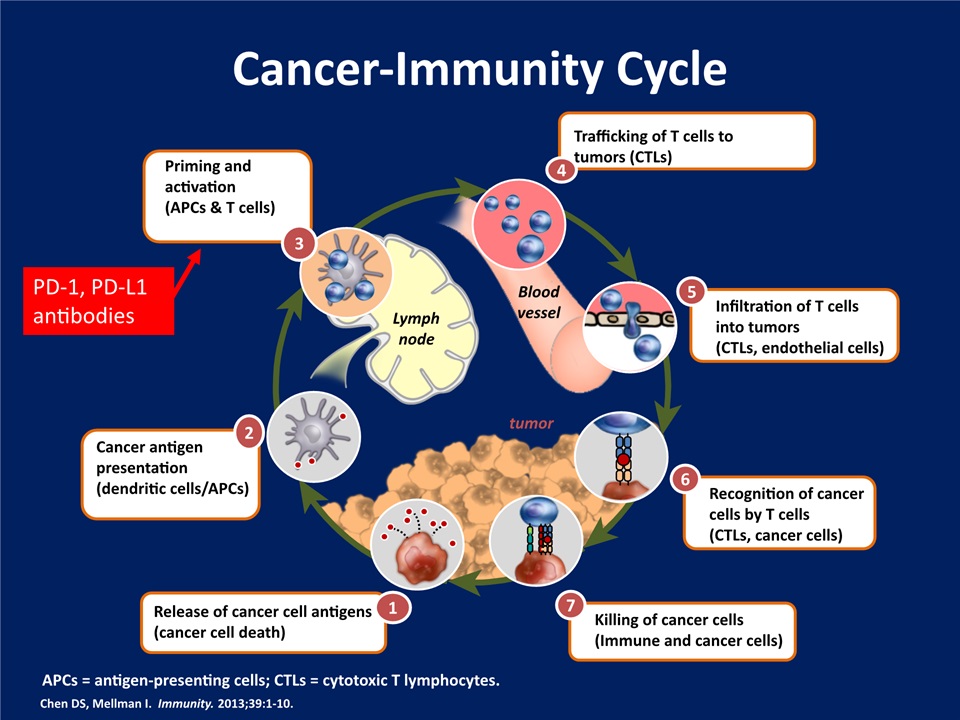
Cancer-Immunity Cycle Chen DS, Mellman I. Immunity. 2013;39:1-10. Release of cancer cell antigens(cancer cell death) 1 Cancer antigen presentation(dendritic cells/APCs) 2 Priming and activation(APCs & T cells) 3 Trafficking of T cells to tumors (CTLs) 4 Infiltration of T cells into tumors(CTLs, endothelial cells) 5 Recognition of cancer cells by T cells(CTLs, cancer cells) 6 Killing of cancer cells(Immune and cancer cells) 7 Lymphnode tumor Bloodvessel APCs = antigen-presenting cells; CTLs = cytotoxic T lymphocytes. PD-1, PD-L1 antibodies
Immune Checkpoints Immune checkpoints are normal ‘brakes’ on the activity of the immune system; checkpoint proteins turn off activated T cells when they are no longer neededImmune checkpoints have been ‘hijacked’ by the cancer to evade the immune systemCheckpoint Inhibitors remove the checkpoint and “take the brakes off the immune system”Now the immune system can ‘see’ the tumor and kill it
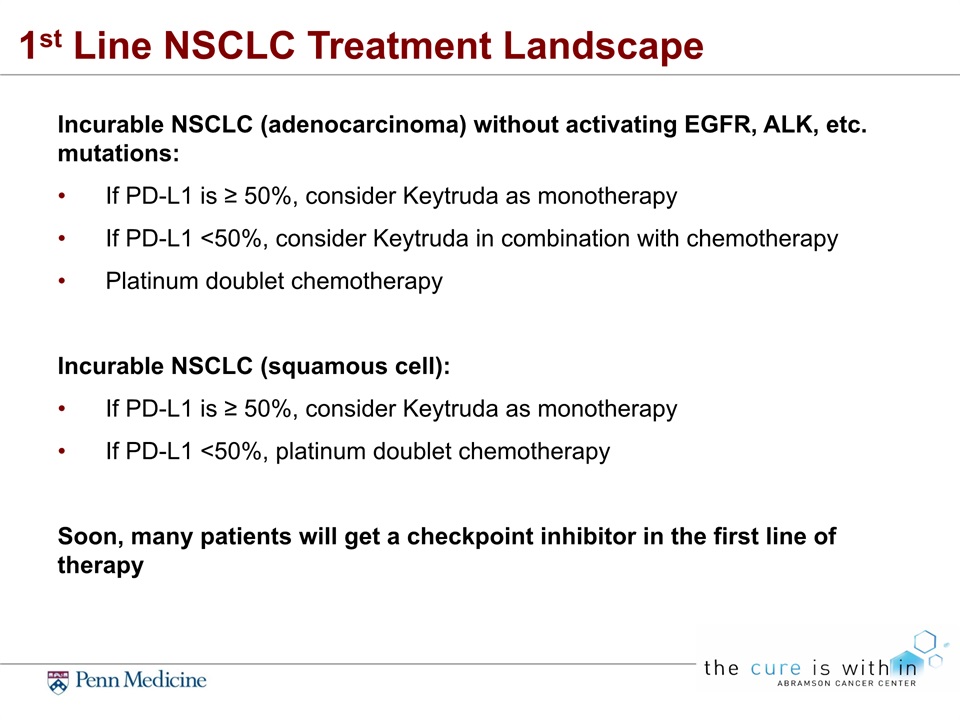
1st Line NSCLC Treatment Landscape Incurable NSCLC (adenocarcinoma) without activating EGFR, ALK, etc. mutations:If PD-L1 is ≥ 50%, consider Keytruda as monotherapyIf PD-L1 <50%, consider Keytruda in combination with chemotherapyPlatinum doublet chemotherapyIncurable NSCLC (squamous cell):If PD-L1 is ≥ 50%, consider Keytruda as monotherapyIf PD-L1 <50%, platinum doublet chemotherapySoon, many patients will get a checkpoint inhibitor in the first line of therapy
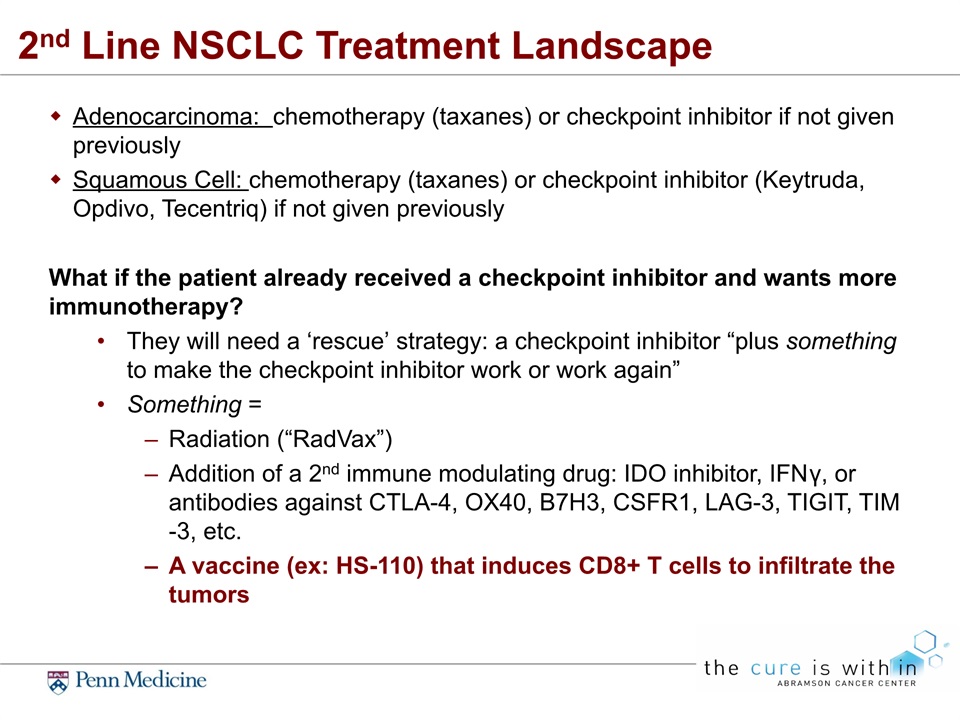
2nd Line NSCLC Treatment Landscape Adenocarcinoma: chemotherapy (taxanes) or checkpoint inhibitor if not given previouslySquamous Cell: chemotherapy (taxanes) or checkpoint inhibitor (Keytruda, Opdivo, Tecentriq) if not given previouslyWhat if the patient already received a checkpoint inhibitor and wants more immunotherapy?They will need a ‘rescue’ strategy: a checkpoint inhibitor “plus something to make the checkpoint inhibitor work or work again”Something = Radiation (“RadVax”)Addition of a 2nd immune modulating drug: IDO inhibitor, IFNγ, or antibodies against CTLA-4, OX40, B7H3, CSFR1, LAG-3, TIGIT, TIM-3, etc.A vaccine (ex: HS-110) that induces CD8+ T cells to infiltrate the tumors

The Big Challenge:Most patients with NSCLC don’t respond to checkpoint inhibition
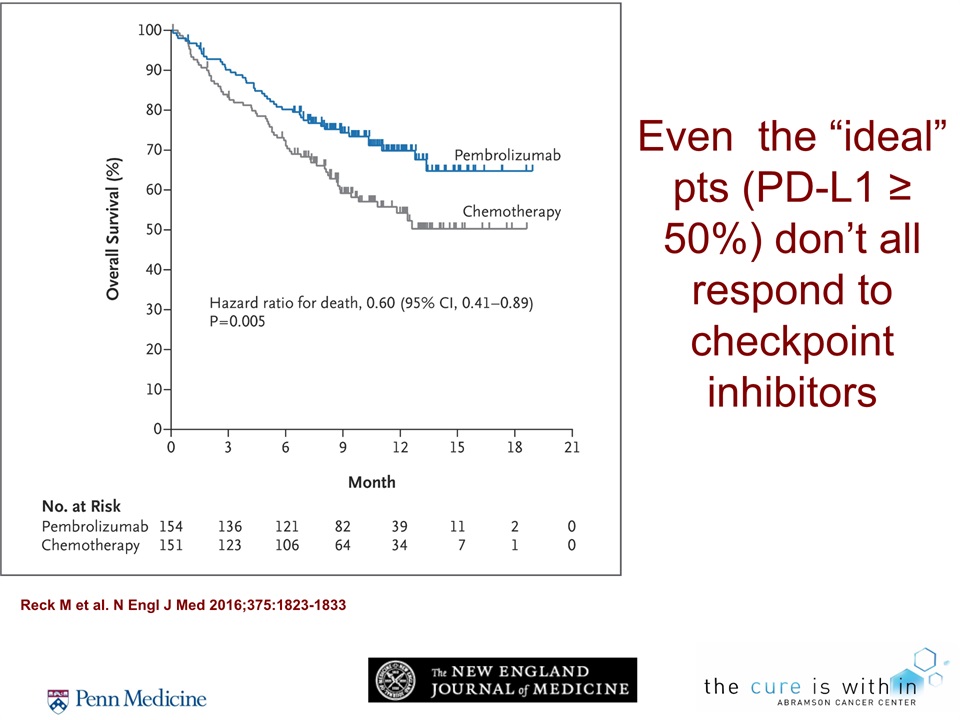
Even the “ideal” pts (PD-L1 ≥ 50%) don’t all respond to checkpoint inhibitors Reck M et al. N Engl J Med 2016;375:1823-1833
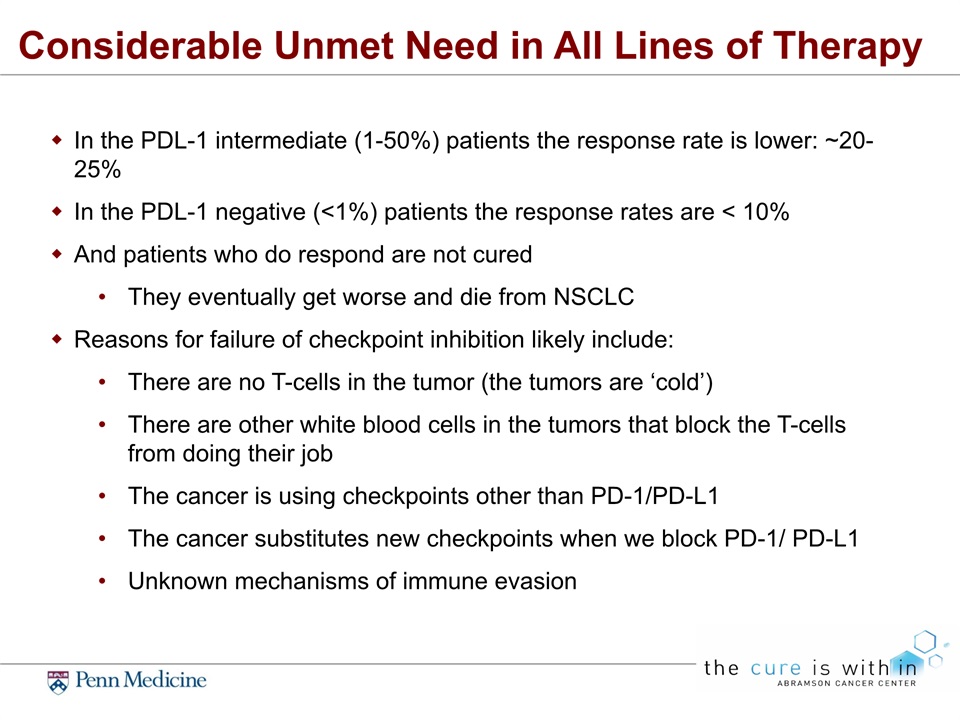
Considerable Unmet Need in All Lines of Therapy In the PDL-1 intermediate (1-50%) patients the response rate is lower: ~20-25% In the PDL-1 negative (<1%) patients the response rates are < 10%And patients who do respond are not curedThey eventually get worse and die from NSCLCReasons for failure of checkpoint inhibition likely include:There are no T-cells in the tumor (the tumors are ‘cold’)There are other white blood cells in the tumors that block the T-cells from doing their jobThe cancer is using checkpoints other than PD-1/PD-L1The cancer substitutes new checkpoints when we block PD-1/ PD-L1Unknown mechanisms of immune evasion
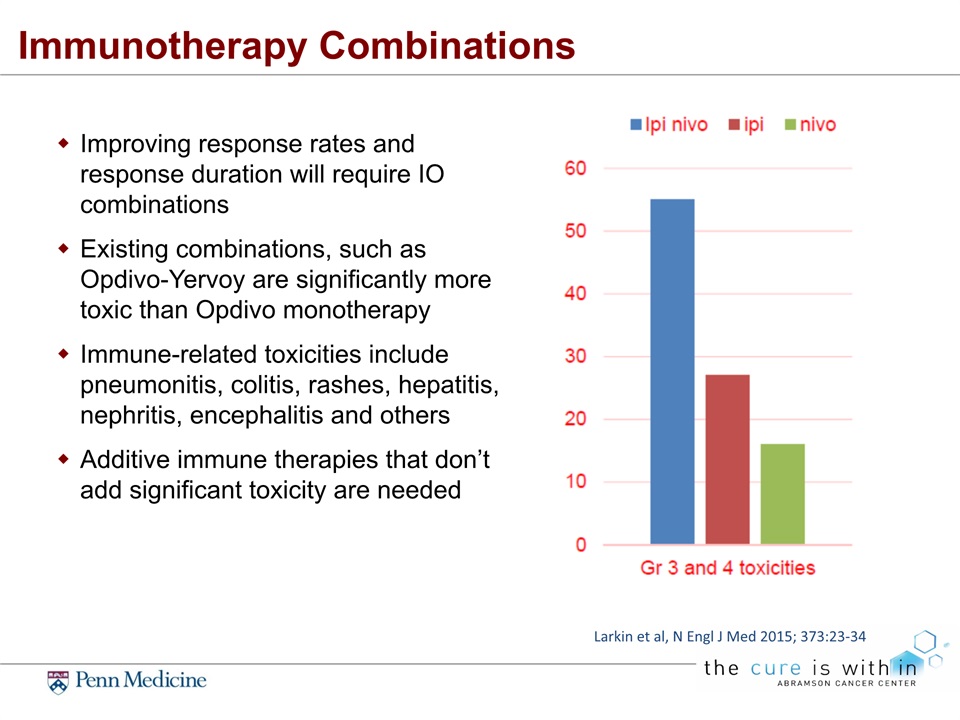
Immunotherapy Combinations Improving response rates and response duration will require IO combinations Existing combinations, such as Opdivo-Yervoy are significantly more toxic than Opdivo monotherapyImmune-related toxicities include pneumonitis, colitis, rashes, hepatitis, nephritis, encephalitis and othersAdditive immune therapies that don’t add significant toxicity are needed Larkin et al, N Engl J Med 2015; 373:23-34
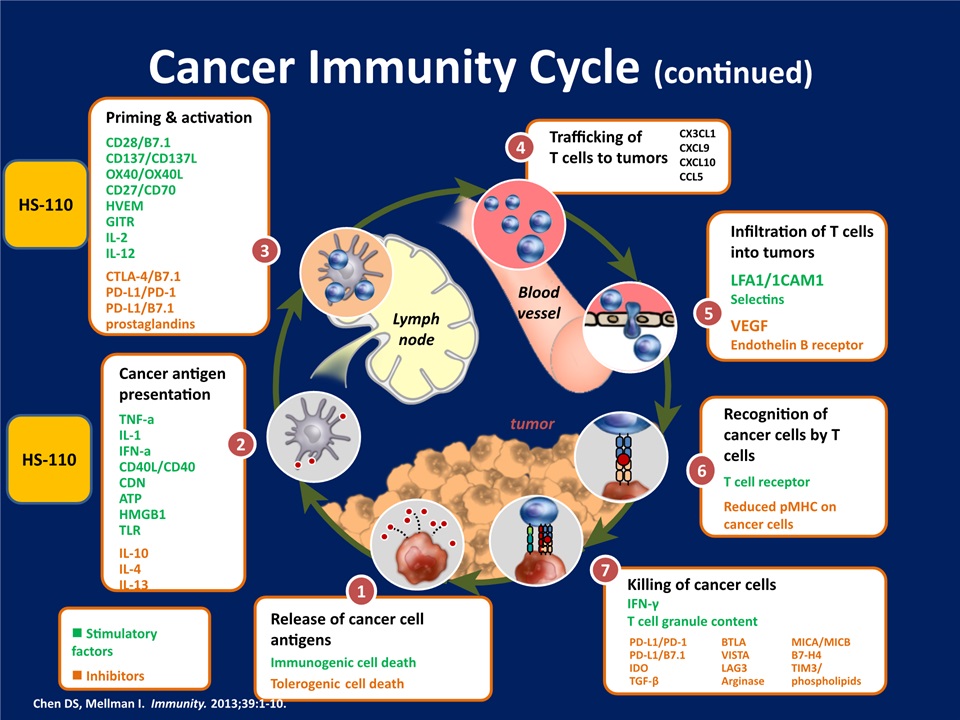
Cancer Immunity Cycle (continued) Infiltration of T cells into tumorsLFA1/1CAM1SelectinsVEGFEndothelin B receptor 5 Trafficking ofT cells to tumors 4 CX3CL1CXCL9CXCL10CCL5 Recognition of cancer cells by T cellsT cell receptorReduced pMHC on cancer cells 6 Killing of cancer cells IFN-γT cell granule content 7 PD-L1/PD-1PD-L1/B7.1IDOTGF-β BTLAVISTALAG3Arginase MICA/MICBB7-H4TIM3/phospholipids Release of cancer cell antigensImmunogenic cell deathTolerogenic cell death 1 n Stimulatory factorsn Inhibitors Cancer antigen presentationTNF-aIL-1IFN-aCD40L/CD40CDNATPHMGB1TLRIL-10IL-4IL-13 2 Priming & activationCD28/B7.1CD137/CD137LOX40/OX40LCD27/CD70HVEMGITRIL-2IL-12CTLA-4/B7.1PD-L1/PD-1PD-L1/B7.1 prostaglandins 3 Lymphnode tumor Bloodvessel Chen DS, Mellman I. Immunity. 2013;39:1-10. HS-110 HS-110
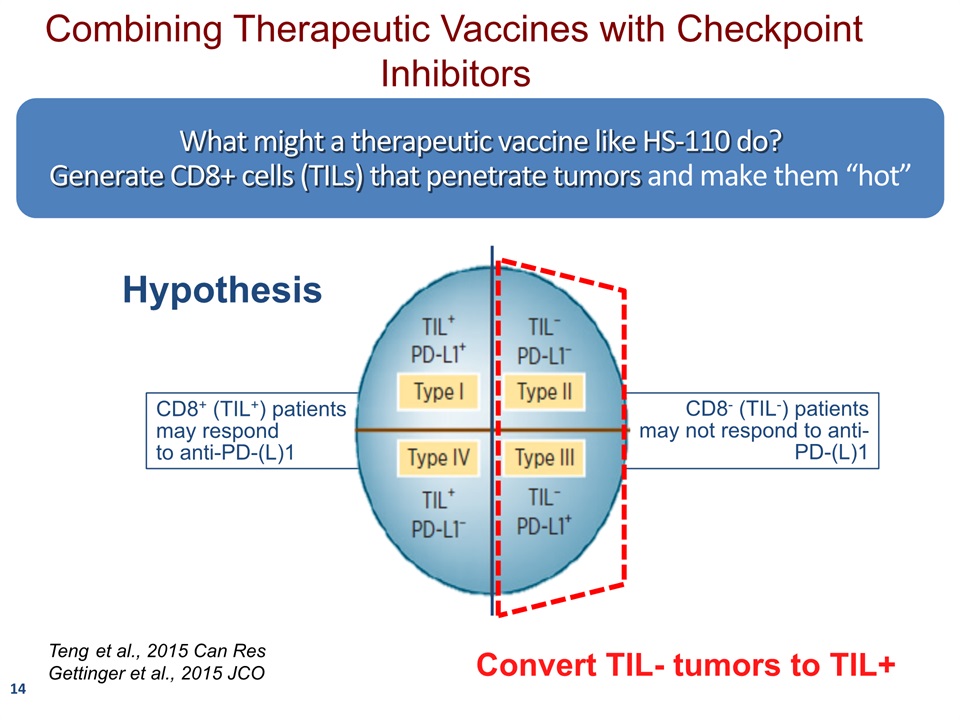
14 What might a therapeutic vaccine like HS-110 do?Generate CD8+ cells (TILs) that penetrate tumors and make them “hot” CD8+ (TIL+) patients may respond to anti-PD-(L)1 CD8- (TIL-) patients may not respond to anti-PD-(L)1 Teng et al., 2015 Can ResGettinger et al., 2015 JCO Hypothesis Combining Therapeutic Vaccines with Checkpoint Inhibitors Convert TIL- tumors to TIL+
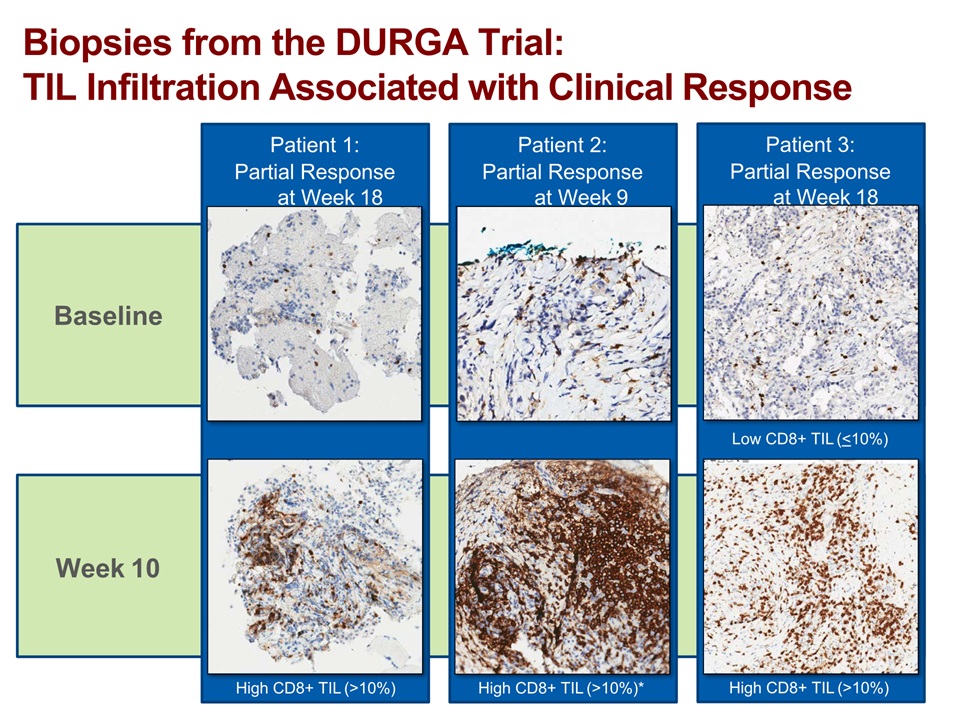
Baseline Week 10 Biopsies from the DURGA Trial:TIL Infiltration Associated with Clinical Response Patient 1:Partial Response at Week 18 High CD8+ TIL (>10%) Patient 2:Partial Response at Week 9 High CD8+ TIL (>10%)* Patient 3:Partial Response at Week 18 Low CD8+ TIL (<10%) High CD8+ TIL (>10%)

Previous lung cancer vaccines were not designed to elicit a robust CD8+ T-cell response
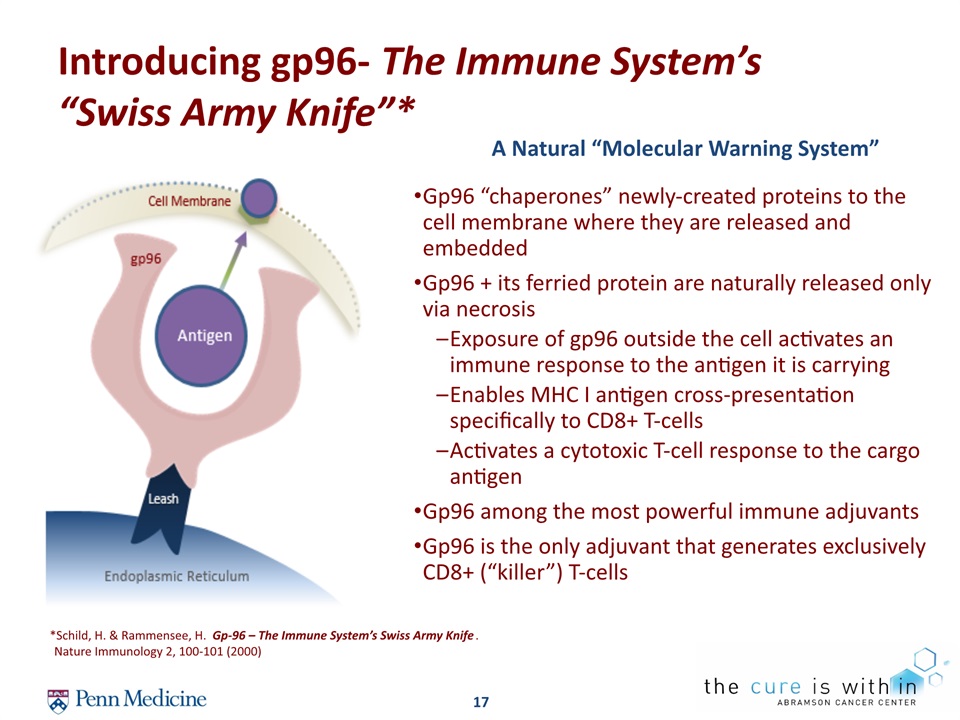
17 Introducing gp96- The Immune System’s “Swiss Army Knife”* *Schild, H. & Rammensee, H. Gp-96 – The Immune System’s Swiss Army Knife. Nature Immunology 2, 100-101 (2000) A Natural “Molecular Warning System” Gp96 “chaperones” newly-created proteins to the cell membrane where they are released and embeddedGp96 + its ferried protein are naturally released only via necrosisExposure of gp96 outside the cell activates an immune response to the antigen it is carrying Enables MHC I antigen cross-presentation specifically to CD8+ T-cellsActivates a cytotoxic T-cell response to the cargo antigenGp96 among the most powerful immune adjuvantsGp96 is the only adjuvant that generates exclusively CD8+ (“killer”) T-cells
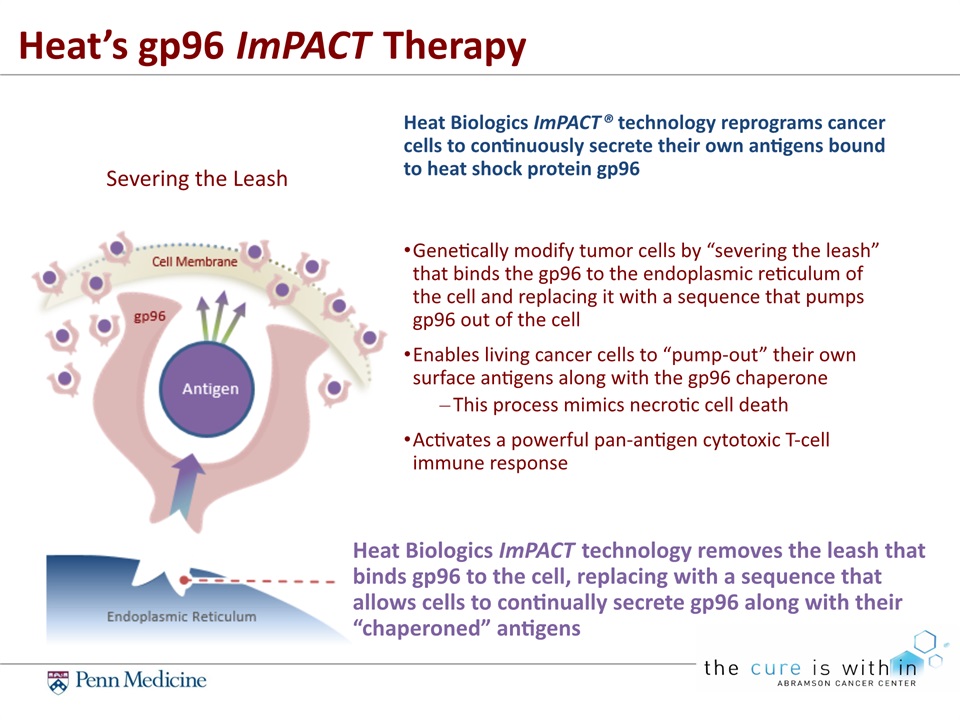
Genetically modify tumor cells by “severing the leash” that binds the gp96 to the endoplasmic reticulum of the cell and replacing it with a sequence that pumps gp96 out of the cellEnables living cancer cells to “pump-out” their own surface antigens along with the gp96 chaperoneThis process mimics necrotic cell deathActivates a powerful pan-antigen cytotoxic T-cell immune response Heat Biologics ImPACT® technology reprograms cancer cells to continuously secrete their own antigens bound to heat shock protein gp96 Heat Biologics ImPACT technology removes the leash that binds gp96 to the cell, replacing with a sequence that allows cells to continually secrete gp96 along with their “chaperoned” antigens Heat’s gp96 ImPACT Therapy Severing the Leash
19 Heat Biologics ImPACT Mechanism of ActionJeff Hutchins PhDChief Scientific OfficerFebruary 28, 2018

20 ImPACT/ComPACT Manufacturing
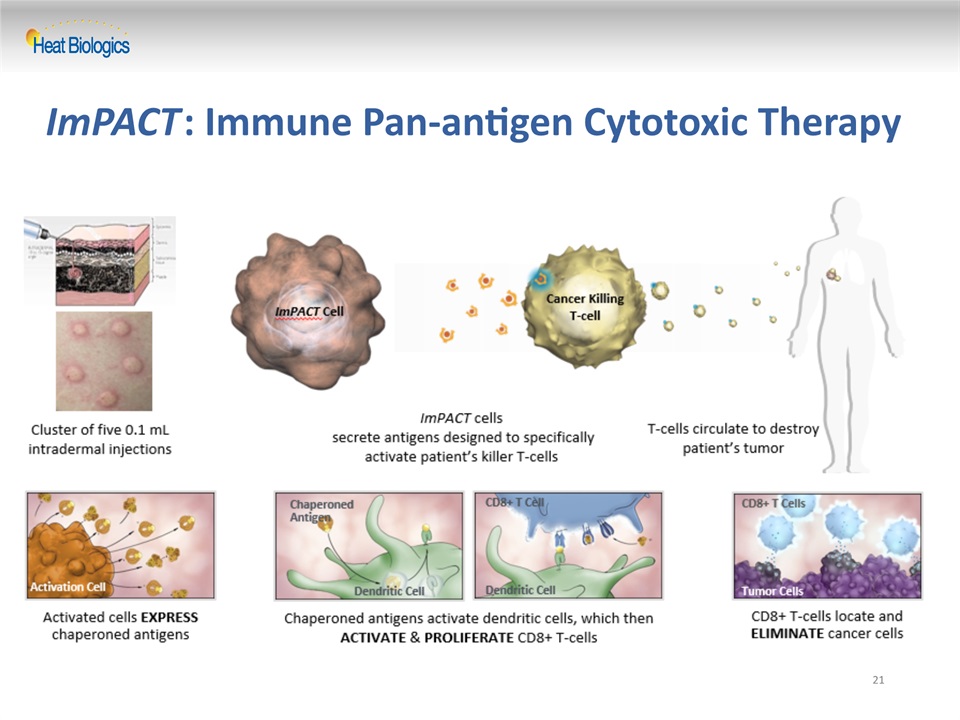
21 ImPACT: Immune Pan-antigen Cytotoxic Therapy
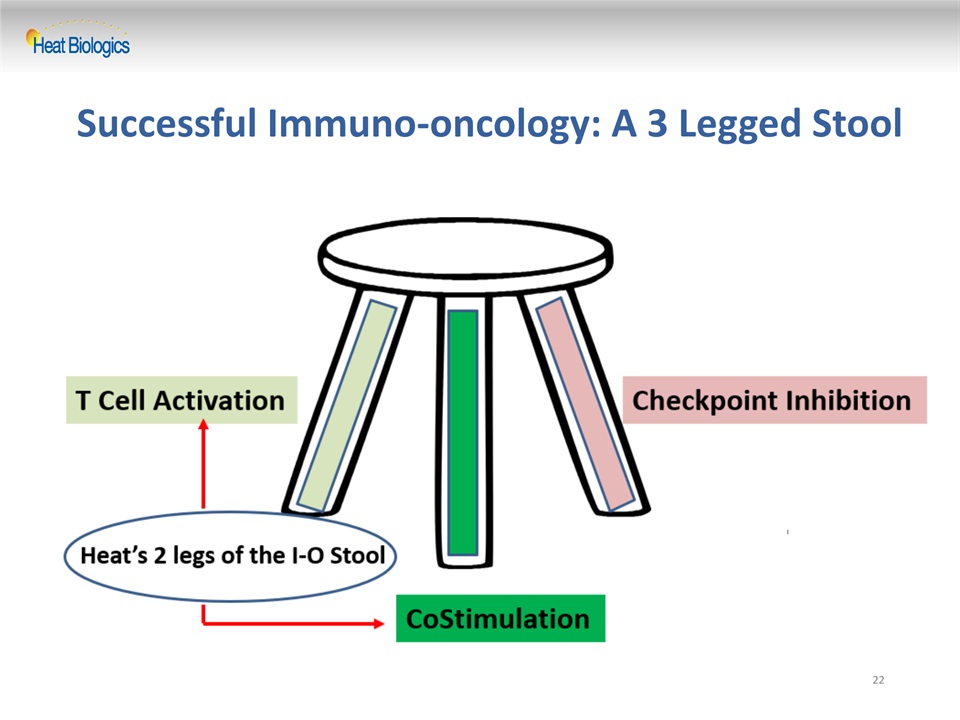
Successful Immuno-oncology: A 3 Legged Stool 22
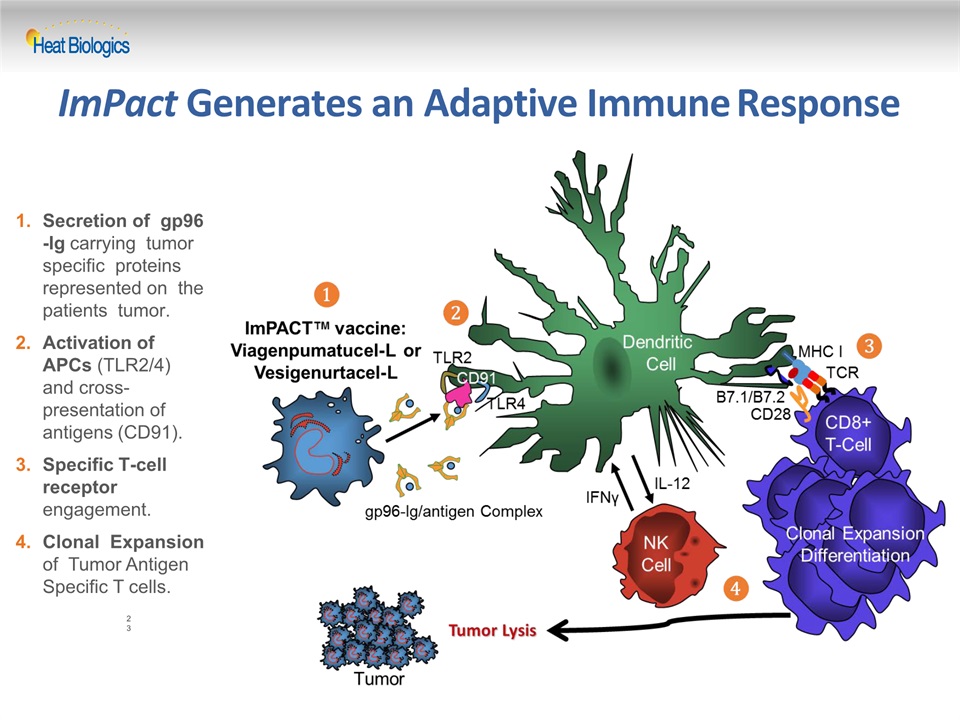
ImPact Generates an Adaptive Immune Response Secretion of gp96-Ig carrying tumor specific proteins represented on the patients tumor.Activation of APCs (TLR2/4) and cross- presentation of antigens (CD91).Specific T-cell receptor engagement.Clonal Expansion of Tumor Antigen Specific T cells. 23
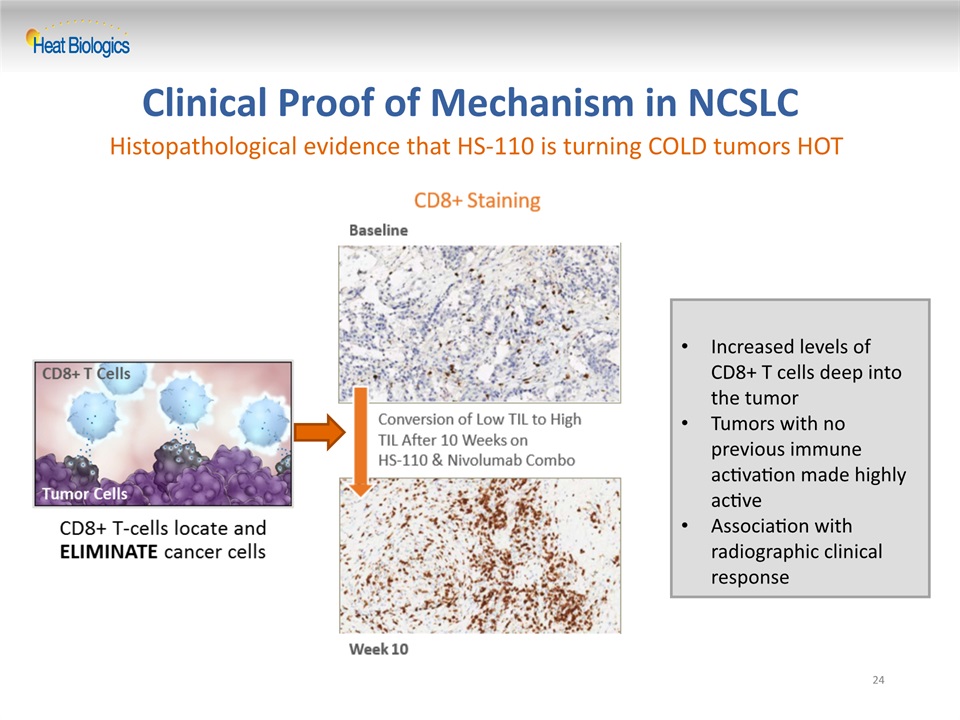
Clinical Proof of Mechanism in NCSLC 24 Increased levels of CD8+ T cells deep into the tumorTumors with no previous immune activation made highly activeAssociation with radiographic clinical response Histopathological evidence that HS-110 is turning COLD tumors HOT
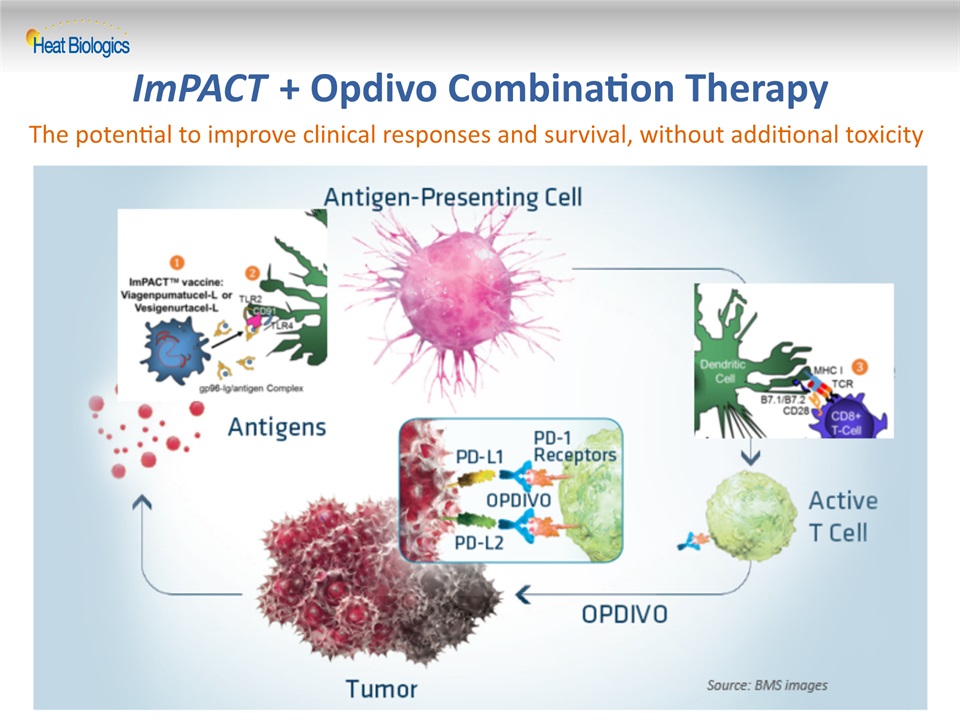
25 ImPACT + Opdivo Combination Therapy The potential to improve clinical responses and survival, without additional toxicity
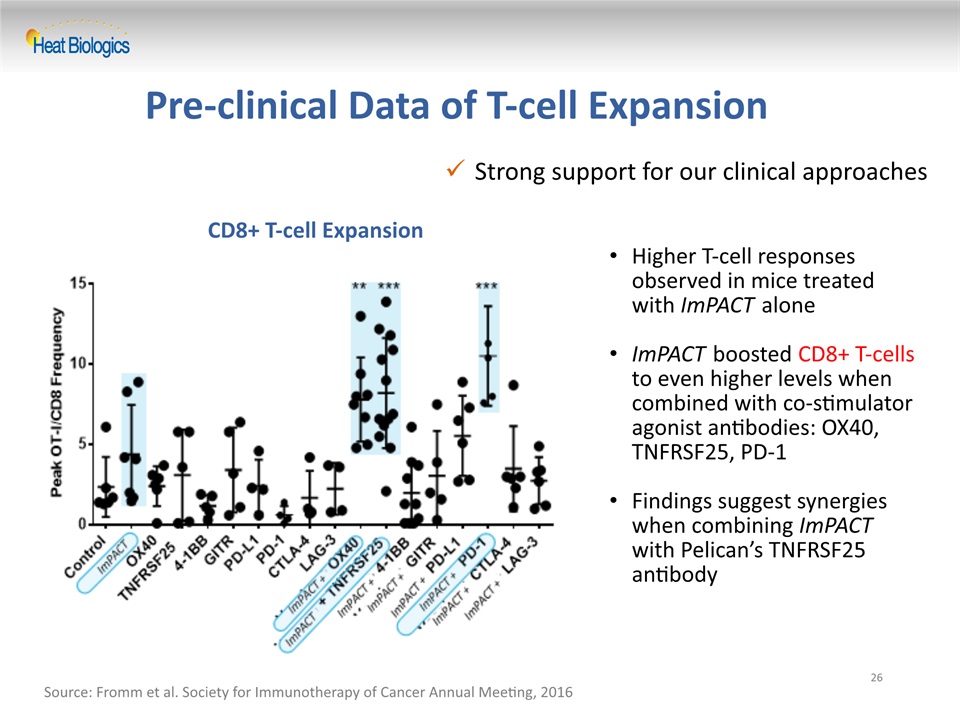
Strong support for our clinical approaches Pre-clinical Data of T-cell Expansion 26 Source: Fromm et al. Society for Immunotherapy of Cancer Annual Meeting, 2016 CD8+ T-cell Expansion Higher T-cell responses observed in mice treated with ImPACT aloneImPACT boosted CD8+ T-cells to even higher levels when combined with co-stimulator agonist antibodies: OX40, TNFRSF25, PD-1Findings suggest synergies when combining ImPACT with Pelican’s TNFRSF25 antibody
27 Heat Biologics DURGA Interim Data ReviewGeorge Peoples MD FACSChief Medical OfficerFebruary 28, 2018
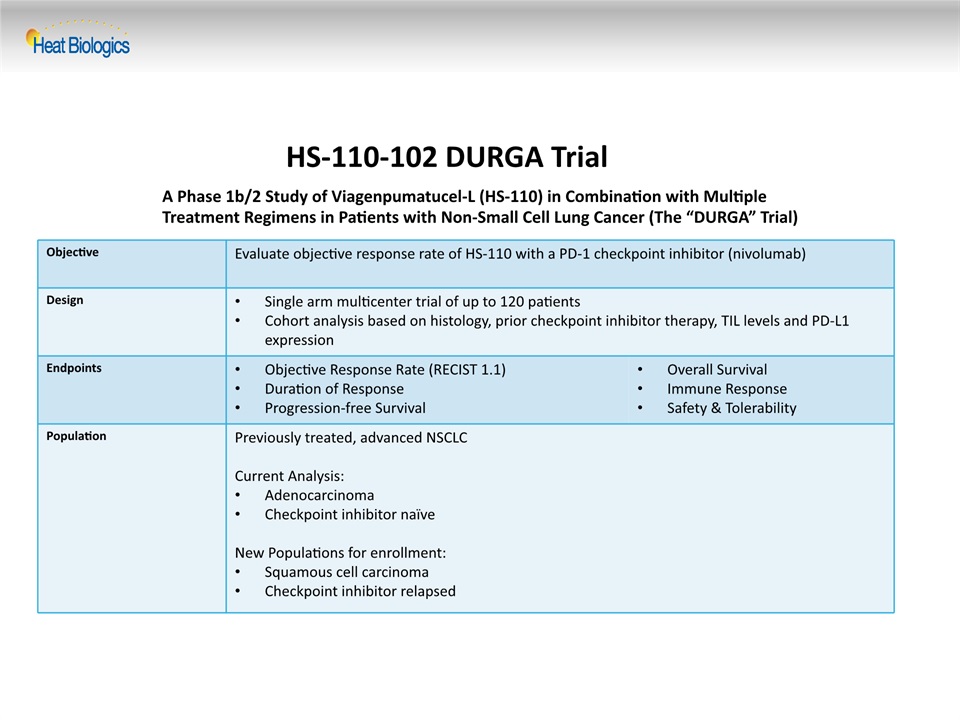
HS-110-102 DURGA Trial Objective Evaluate objective response rate of HS-110 with a PD-1 checkpoint inhibitor (nivolumab) Design Single arm multicenter trial of up to 120 patientsCohort analysis based on histology, prior checkpoint inhibitor therapy, TIL levels and PD-L1 expression Endpoints Objective Response Rate (RECIST 1.1)Duration of ResponseProgression-free Survival Overall SurvivalImmune ResponseSafety & Tolerability Population Previously treated, advanced NSCLCCurrent Analysis:AdenocarcinomaCheckpoint inhibitor naïveNew Populations for enrollment:Squamous cell carcinomaCheckpoint inhibitor relapsed A Phase 1b/2 Study of Viagenpumatucel-L (HS-110) in Combination with Multiple Treatment Regimens in Patients with Non-Small Cell Lung Cancer (The “DURGA” Trial)
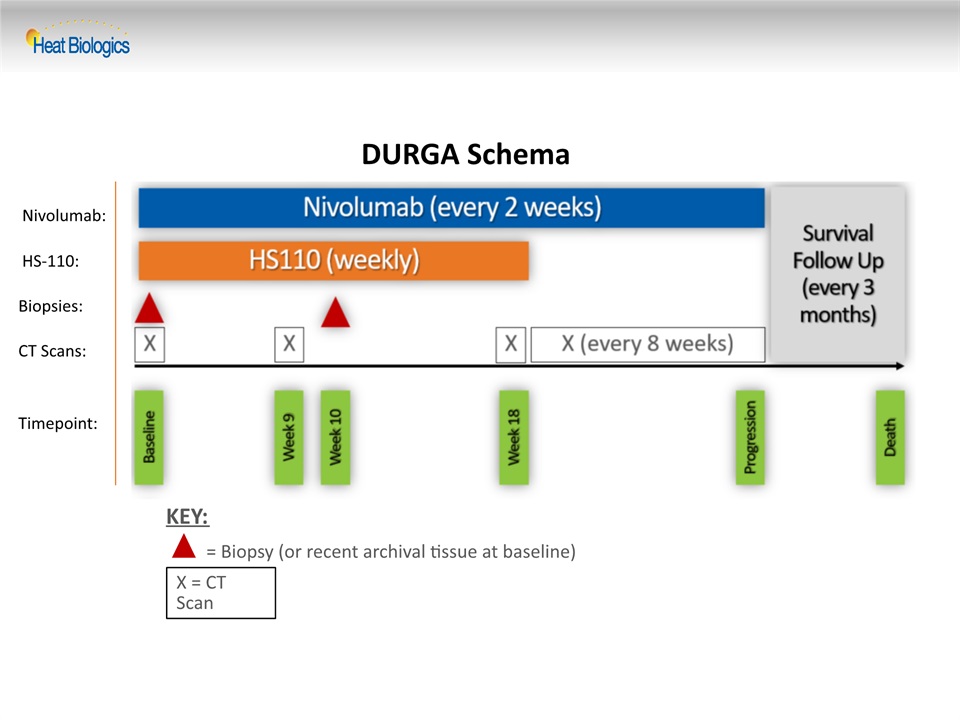
DURGA Schema X = CT Scan = Biopsy (or recent archival tissue at baseline) KEY: Nivolumab: HS-110: Biopsies: CT Scans: Timepoint:
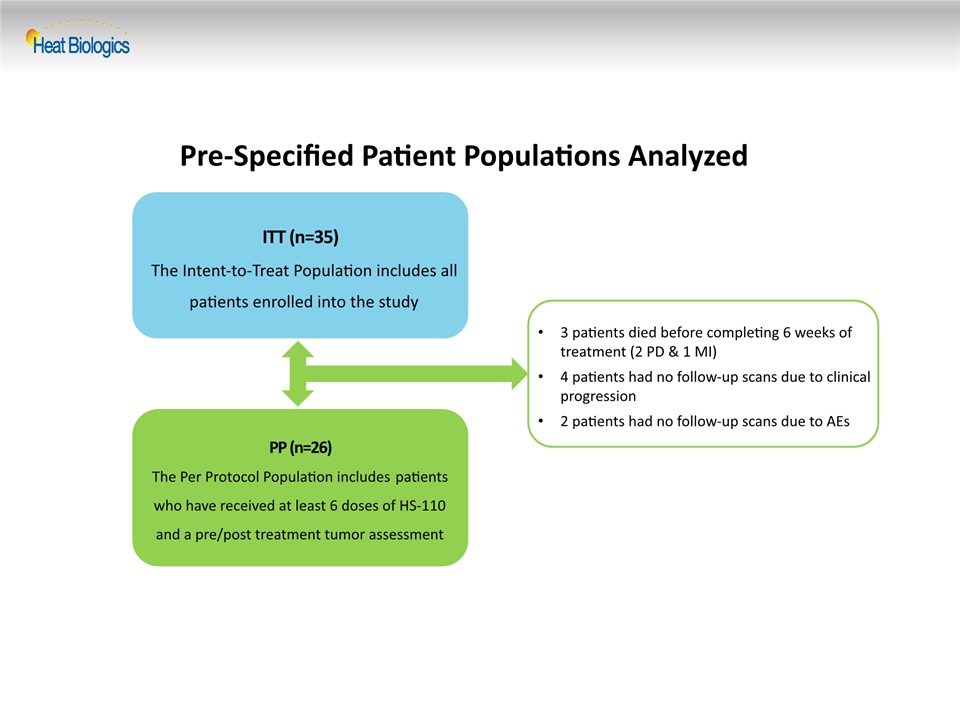
Pre-Specified Patient Populations Analyzed ITT (n=35)The Intent-to-Treat Population includes all patients enrolled into the study PP (n=26)The Per Protocol Population includes patients who have received at least 6 doses of HS-110 and a pre/post treatment tumor assessment 3 patients died before completing 6 weeks of treatment (2 PD & 1 MI)4 patients had no follow-up scans due to clinical progression2 patients had no follow-up scans due to AEs
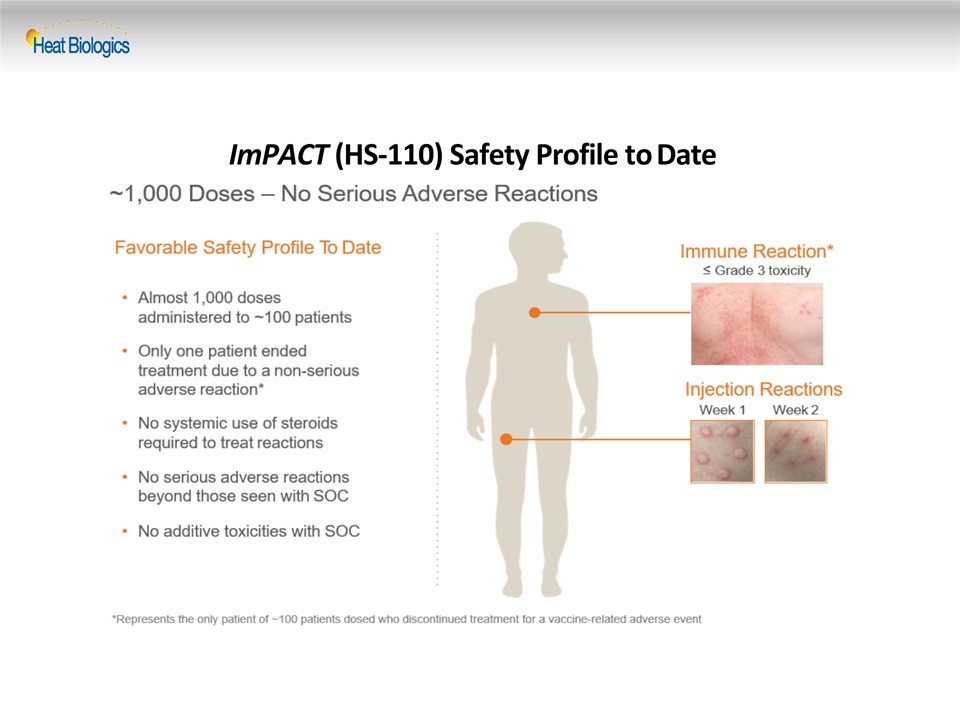
ImPACT (HS-110) Safety Profile to Date
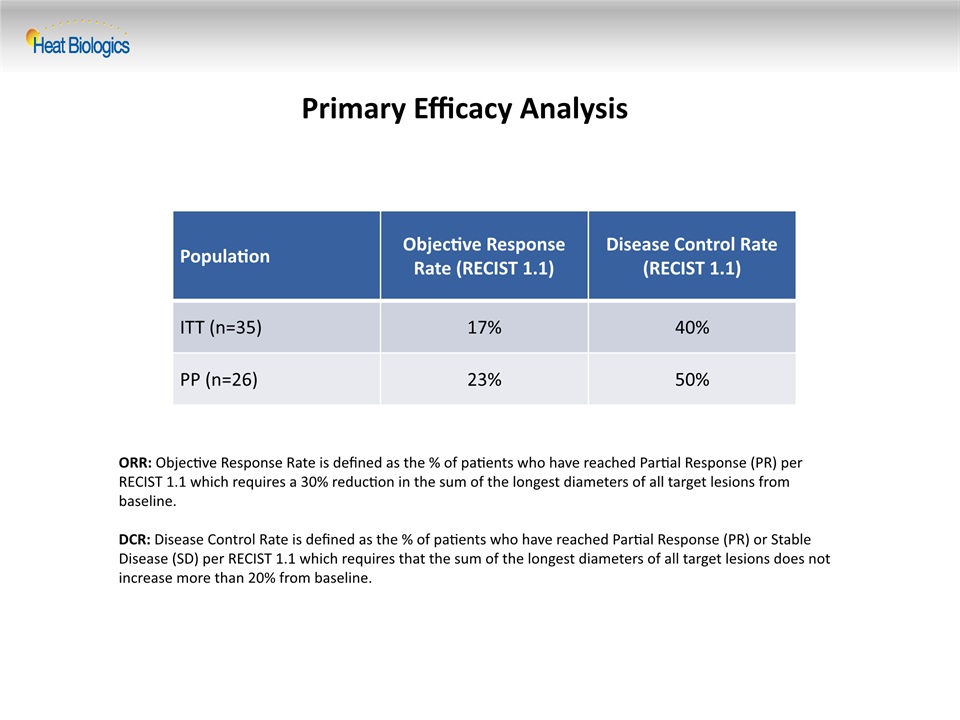
Primary Efficacy Analysis Population Objective Response Rate (RECIST 1.1) Disease Control Rate (RECIST 1.1) ITT (n=35) 17% 40% PP (n=26) 23% 50% ORR: Objective Response Rate is defined as the % of patients who have reached Partial Response (PR) per RECIST 1.1 which requires a 30% reduction in the sum of the longest diameters of all target lesions from baseline.DCR: Disease Control Rate is defined as the % of patients who have reached Partial Response (PR) or Stable Disease (SD) per RECIST 1.1 which requires that the sum of the longest diameters of all target lesions does not increase more than 20% from baseline.
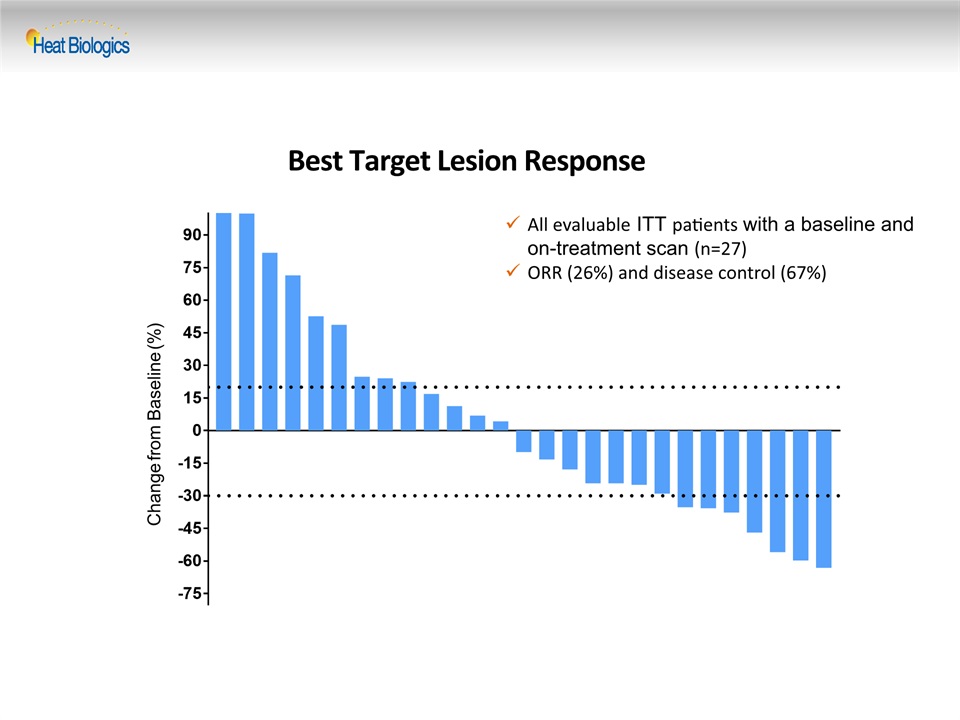
Best Target Lesion Response Change from Baseline (%) All evaluable ITT patients with a baseline and on-treatment scan (n=27) ORR (26%) and disease control (67%)
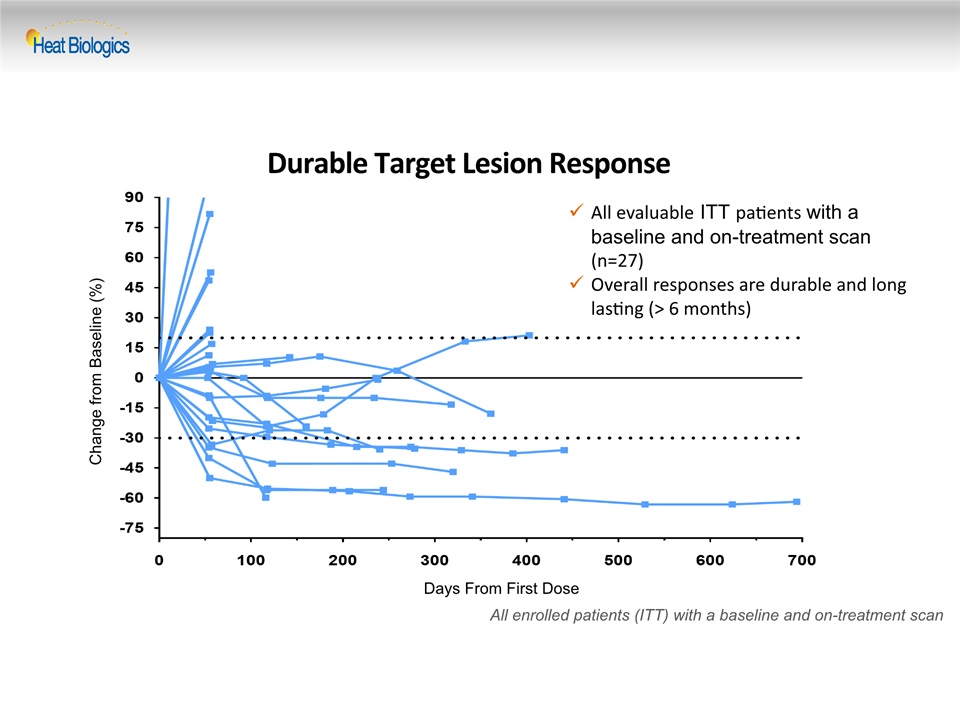
Durable Target Lesion Response Change from Baseline (%) All enrolled patients (ITT) with a baseline and on-treatment scan Days From First Dose All evaluable ITT patients with a baseline and on-treatment scan (n=27) Overall responses are durable and long lasting (> 6 months)
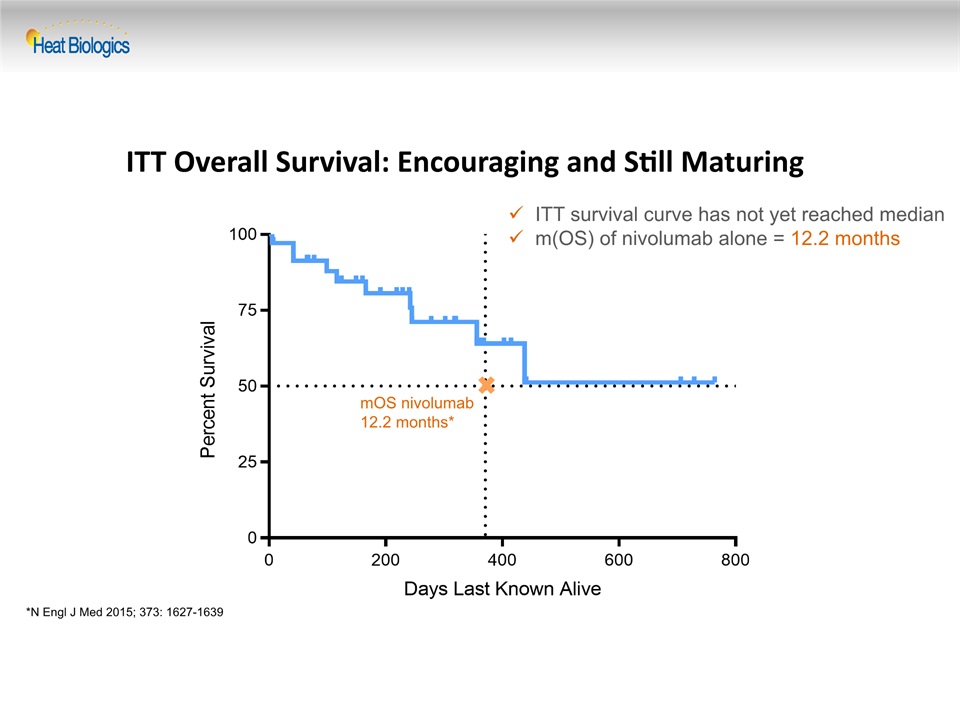
ITT Overall Survival: Encouraging and Still Maturing ITT survival curve has not yet reached medianm(OS) of nivolumab alone = 12.2 months mOS nivolumab12.2 months* *N Engl J Med 2015; 373: 1627-1639
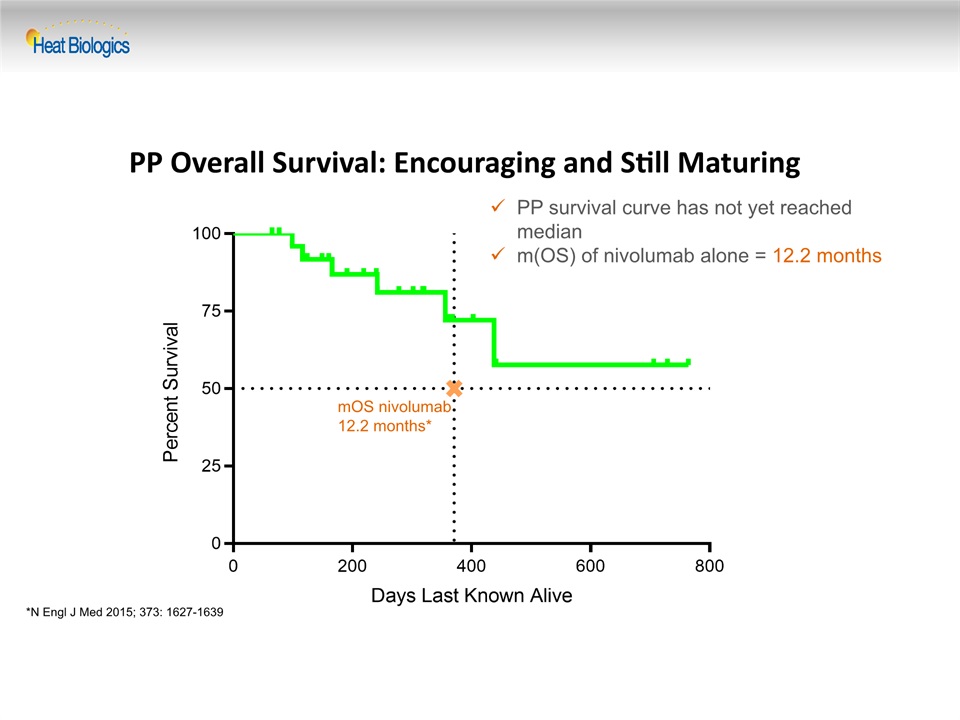
PP Overall Survival: Encouraging and Still Maturing PP survival curve has not yet reached medianm(OS) of nivolumab alone = 12.2 months mOS nivolumab12.2 months* *N Engl J Med 2015; 373: 1627-1639
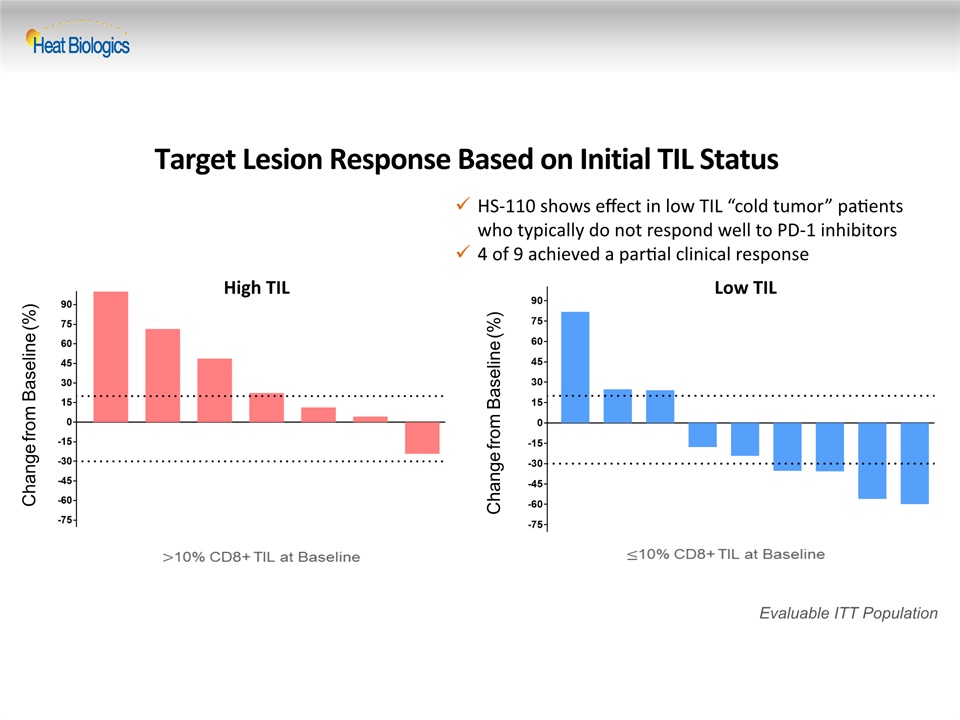
Target Lesion Response Based on Initial TIL Status Change from Baseline (%) Evaluable ITT Population Change from Baseline (%) High TIL Low TIL HS-110 shows effect in low TIL “cold tumor” patients who typically do not respond well to PD-1 inhibitors4 of 9 achieved a partial clinical response
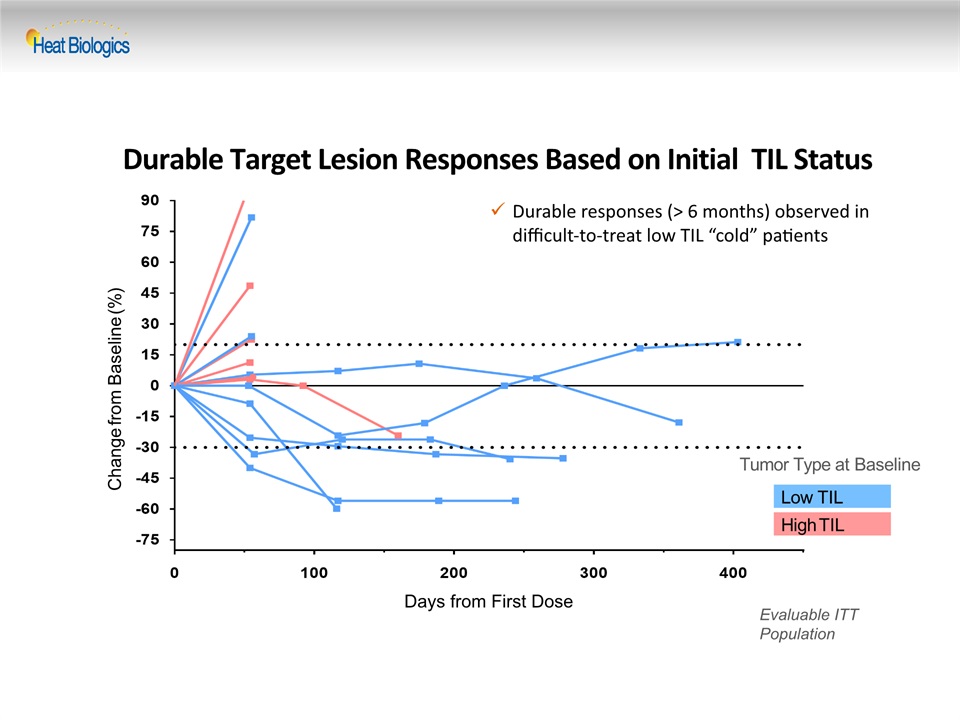
Durable Target Lesion Responses Based on Initial TIL Status Low TIL High TIL Change from Baseline (%) Tumor Type at Baseline Evaluable ITT Population Days from First Dose Durable responses (> 6 months) observed in difficult-to-treat low TIL “cold” patients
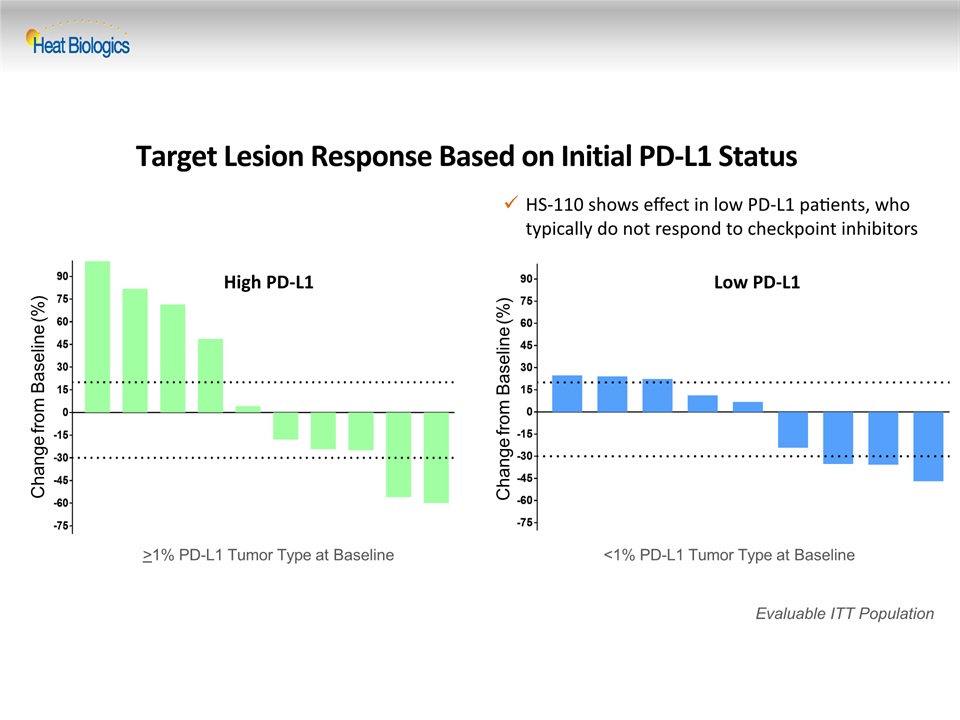
Target Lesion Response Based on Initial PD-L1 Status Change from Baseline (%) Evaluable ITT Population Change from Baseline (%) >1% PD-L1 Tumor Type at Baseline <1% PD-L1 Tumor Type at Baseline High PD-L1 Low PD-L1 HS-110 shows effect in low PD-L1 patients, who typically do not respond to checkpoint inhibitors

Durable Target Lesion Responses Based on Initial PD-L1 Status Change from Baseline (%) Evaluable ITT Population Days from First Dose Change from Baseline (%) Days from First Dose >1% PD-L1 Tumor Type at Baseline <1% PD-L1 Tumor Type at Baseline Durable responses observed in difficult-to-treat low PD-L1 patients High PD-L1 Low PD-L1
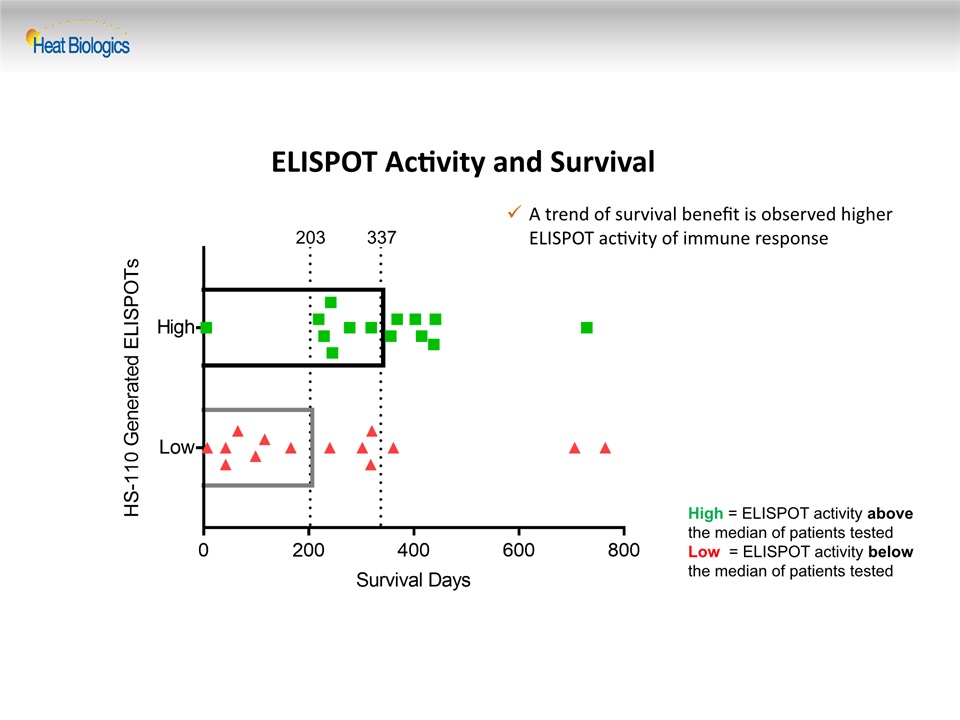
ELISPOT Activity and Survival High = ELISPOT activity above the median of patients tested Low = ELISPOT activity below the median of patients tested A trend of survival benefit is observed higher ELISPOT activity of immune response 203 337
Summary of Interim Data Tumor shrinkage and disease control demonstrated in a majority of evaluable patientsOverall responses are durable and long lastingWhile survival data is still maturing, the median overall survival has not yet been reachedHS-110 shows durable responses in difficult-to-treat low TIL “cold tumor” patientsHS110 shows durable responses in low PD-L1 patients, who typically do not respond to checkpoint inhibitorsA trend of survival benefit is observed with higher ELISPOT activity reflective of tumor antigen-specific immune response This data is consistent with HS-110 mechanism of action as well as data previously reported in our phase 1 trial