INVESTOR PRESENTATION
Published on January 8, 2019
EXHIBIT 99.1
Heat Biologics Corporate PresentationJanuary 2019

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2017 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2
To activate CD8+ “Killer” T-cells to turn “COLD” tumors “HOT” 3 Our Mission We seek to combine with checkpoint inhibitors and other immunotherapies to dramatically increase their effectiveness 3

Investment Highlights Potential Best in Class Oncology Treatment - T-cell activating platform (TCAP) produces allogeneic, off-the-shelf therapies designed to activate the immune system to turn immunologically “cold” tumors “hot”Combination Effect - Our therapies are designed to be administered with checkpoint inhibitors and other immuno-modulators to enhance immune responseOff-the-shelf Therapies - We can administer drug immediately without extracting patient material, simplifying treatment and substantially lowering cost of goods soldClinical Data with Checkpoint Inhibitors - Positive interim data from ongoing Phase 2 trial of HS-110 + checkpoint inhibitor in non-small cell lung cancer (NSCLC)Diverse Technology Platforms - Multiple complementary platform technologiesStrong Management Team - Senior team with broad experience in biotech, pharma, clinical development and research 4
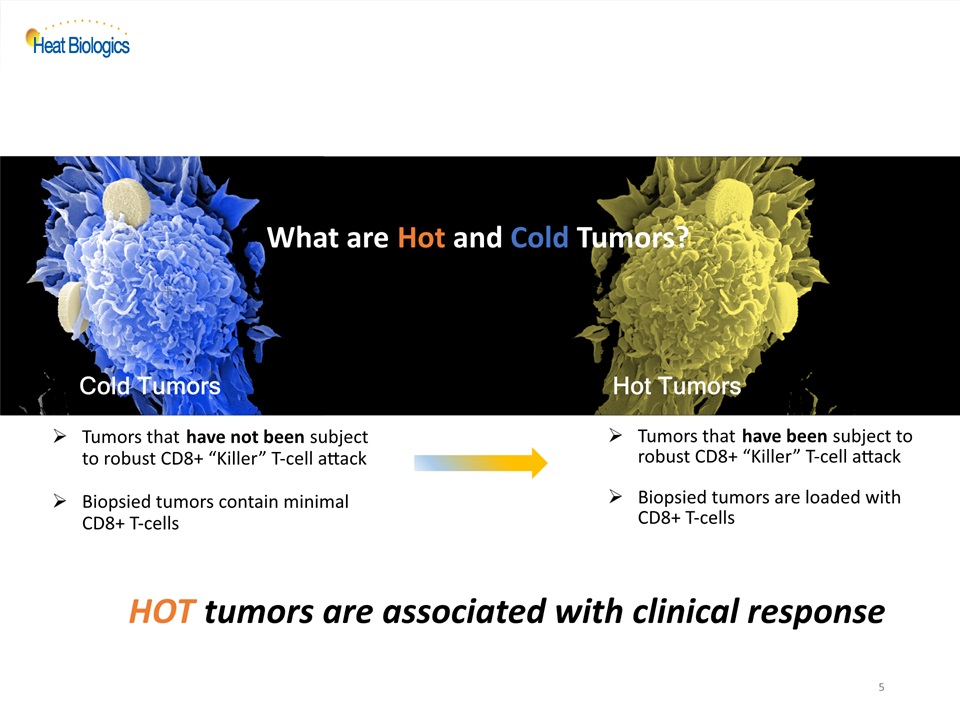
5 Cold Tumors Tumors that have not been subject to robust CD8+ “Killer” T-cell attackBiopsied tumors contain minimal CD8+ T-cells Hot Tumors Tumors that have been subject to robust CD8+ “Killer” T-cell attack Biopsied tumors are loaded with CD8+ T-cells HOT tumors are associated with clinical response What are Hot and Cold Tumors?
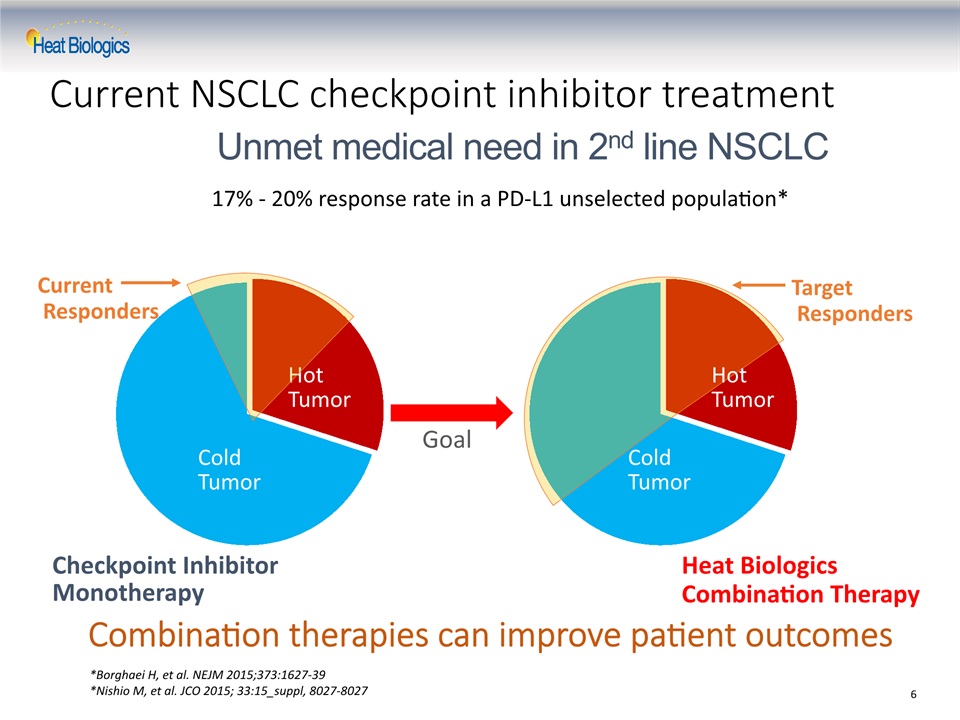
Current NSCLC checkpoint inhibitor treatment Checkpoint Inhibitor Monotherapy Heat BiologicsCombination Therapy Cold Tumor Cold Tumor Hot Tumor Hot Tumor Current Responders Target Responders Goal Unmet medical need in 2nd line NSCLC Combination therapies can improve patient outcomes 6 17% - 20% response rate in a PD-L1 unselected population* *Borghaei H, et al. NEJM 2015;373:1627-39*Nishio M, et al. JCO 2015; 33:15_suppl, 8027-8027
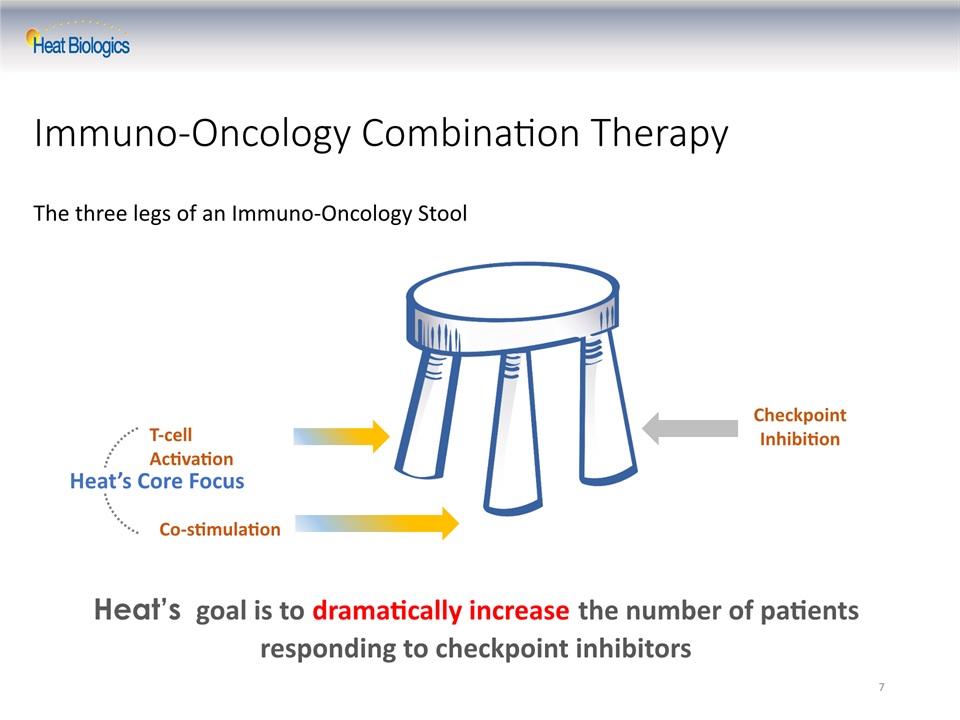
7 Immuno-Oncology Combination Therapy The three legs of an Immuno-Oncology Stool T-cell Activation Co-stimulation Checkpoint Inhibition Heat’s Core Focus Heat’s goal is to dramatically increase the number of patients responding to checkpoint inhibitors
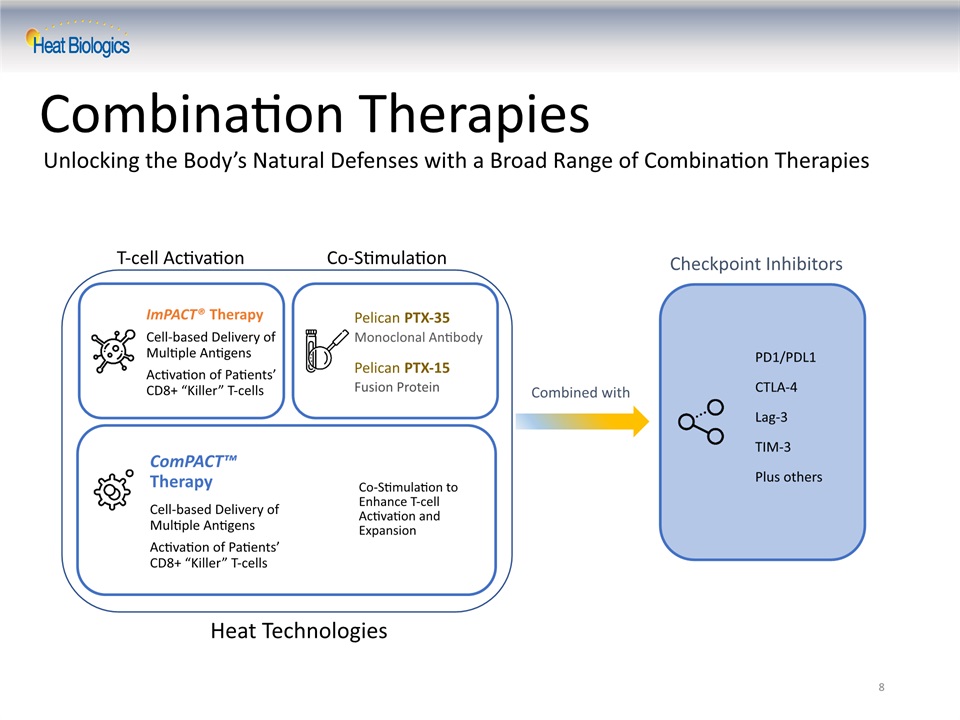
Unlocking the Body’s Natural Defenses with a Broad Range of Combination Therapies 8 Combination Therapies T-cell Activation Co-Stimulation Pelican PTX-35Monoclonal AntibodyPelican PTX-15Fusion Protein Checkpoint Inhibitors PD1/PDL1CTLA-4Lag-3TIM-3Plus others Combined with ImPACT® TherapyCell-based Delivery of Multiple Antigens Activation of Patients’CD8+ “Killer” T-cells ComPACT™ TherapyCell-based Delivery ofMultiple Antigens Activation of Patients’CD8+ “Killer” T-cells Co-Stimulation to Enhance T-cell Activation and Expansion Heat Technologies
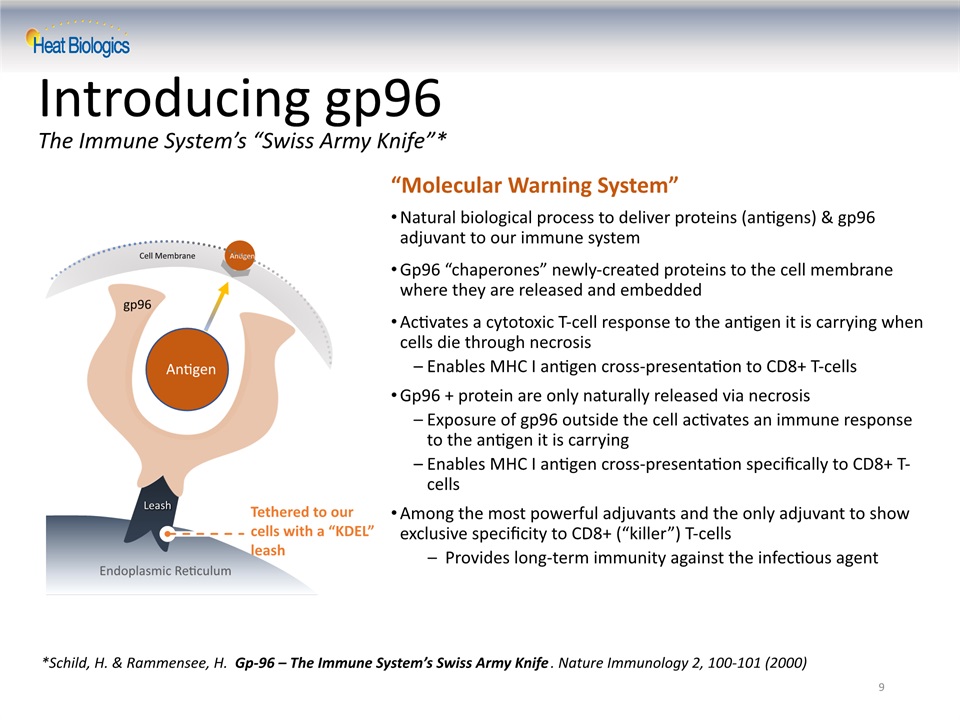
9 Introducing gp96 The Immune System’s “Swiss Army Knife”* *Schild, H. & Rammensee, H. Gp-96 – The Immune System’s Swiss Army Knife. Nature Immunology 2, 100-101 (2000) “Molecular Warning System” Natural biological process to deliver proteins (antigens) & gp96 adjuvant to our immune systemGp96 “chaperones” newly-created proteins to the cell membrane where they are released and embeddedActivates a cytotoxic T-cell response to the antigen it is carrying when cells die through necrosisEnables MHC I antigen cross-presentation to CD8+ T-cellsGp96 + protein are only naturally released via necrosisExposure of gp96 outside the cell activates an immune response to the antigen it is carrying Enables MHC I antigen cross-presentation specifically to CD8+ T-cellsAmong the most powerful adjuvants and the only adjuvant to show exclusive specificity to CD8+ (“killer”) T-cellsProvides long-term immunity against the infectious agent Tethered to our cells with a “KDEL” leash gp96 antigen Leash Endoplasmic Reticulum Cell Membrane Antigen Antigen
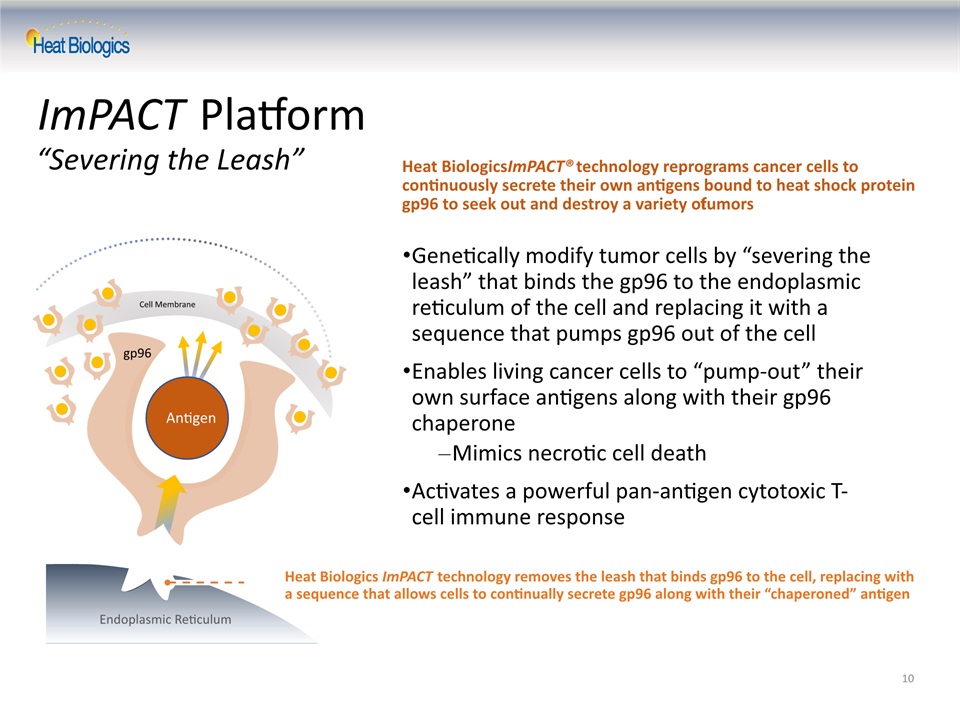
Genetically modify tumor cells by “severing the leash” that binds the gp96 to the endoplasmic reticulum of the cell and replacing it with a sequence that pumps gp96 out of the cellEnables living cancer cells to “pump-out” their own surface antigens along with their gp96 chaperoneMimics necrotic cell deathActivates a powerful pan-antigen cytotoxic T-cell immune response Heat Biologics ImPACT® technology reprograms cancer cells to continuously secrete their own antigens bound to heat shock protein gp96 to seek out and destroy a variety of tumors Heat Biologics ImPACT technology removes the leash that binds gp96 to the cell, replacing with a sequence that allows cells to continually secrete gp96 along with their “chaperoned” antigen 10 ImPACT Platform “Severing the Leash” gp96 antigen Leash Endoplasmic Reticulum Cell Membrane Antigen
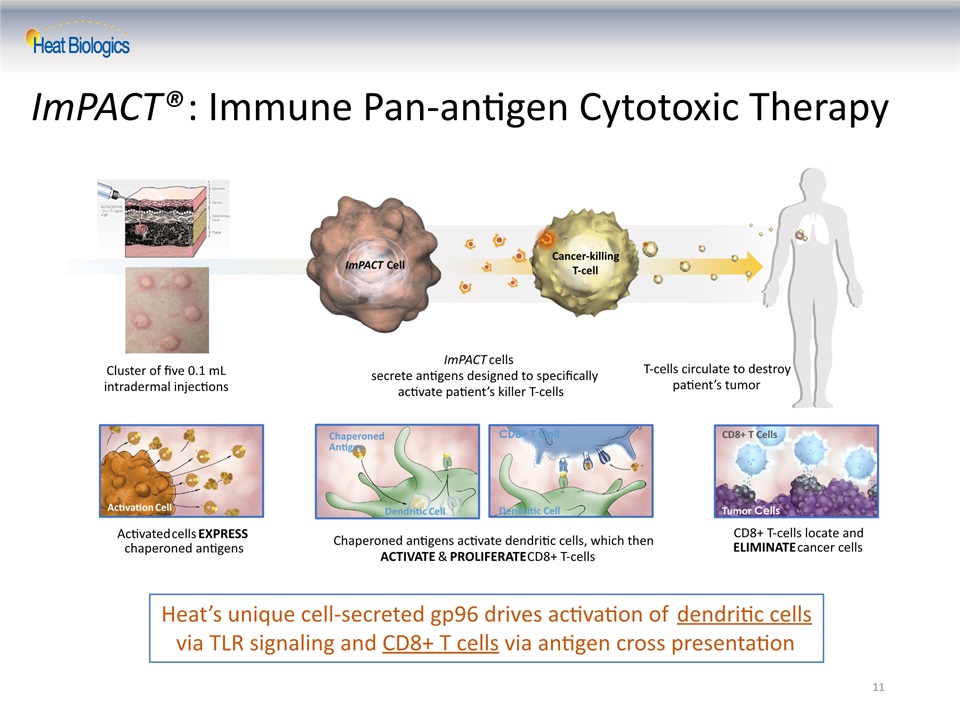
11 ImPACT®: Immune Pan-antigen Cytotoxic Therapy ImPACT Cell Cancer-killing T-cell ImPACT cells secrete antigens designed to specifically activate patient’s killer T-cells T-cells circulate to destroy patient’s tumor Cluster of five 0.1 mL intradermal injections Activated cells EXPRESS chaperoned antigens Activation Cell CD8+ T Cell Dendritic Cell Chaperoned antigens activate dendritic cells, which thenACTIVATE & PROLIFERATE CD8+ T-cells Dendritic Cell ChaperonedAntigen CD8+ T-cells locate and ELIMINATE cancer cells Tumor Cells CD8+ T Cells Heat’s unique cell-secreted gp96 drives activation of dendritic cells via TLR signaling and CD8+ T cells via antigen cross presentation
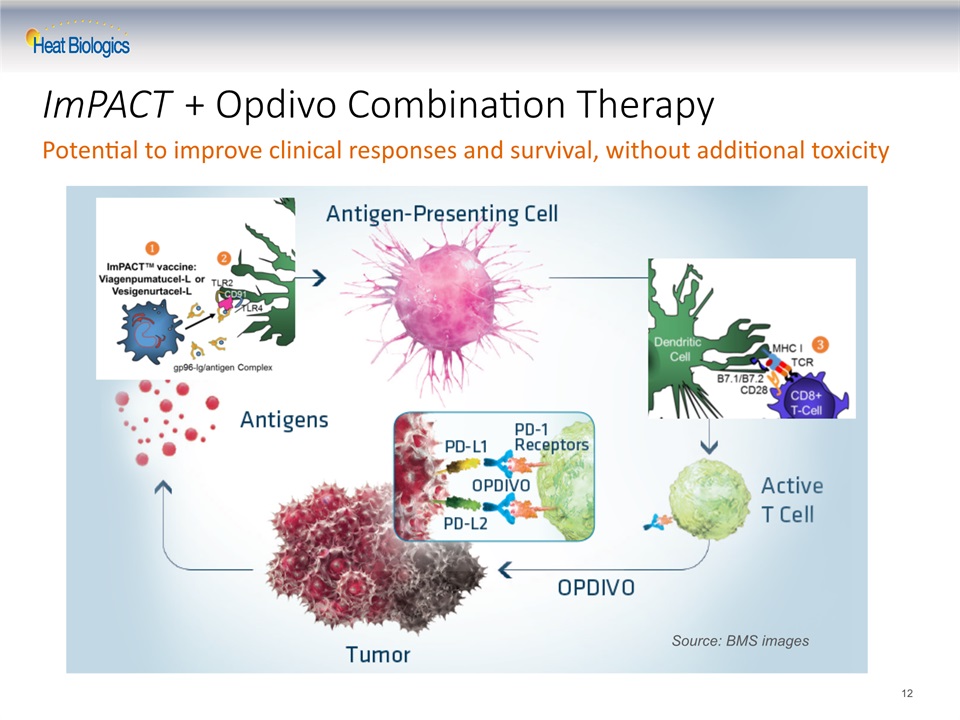
ImPACT + Opdivo Combination Therapy 12 Source: BMS images Potential to improve clinical responses and survival, without additional toxicity
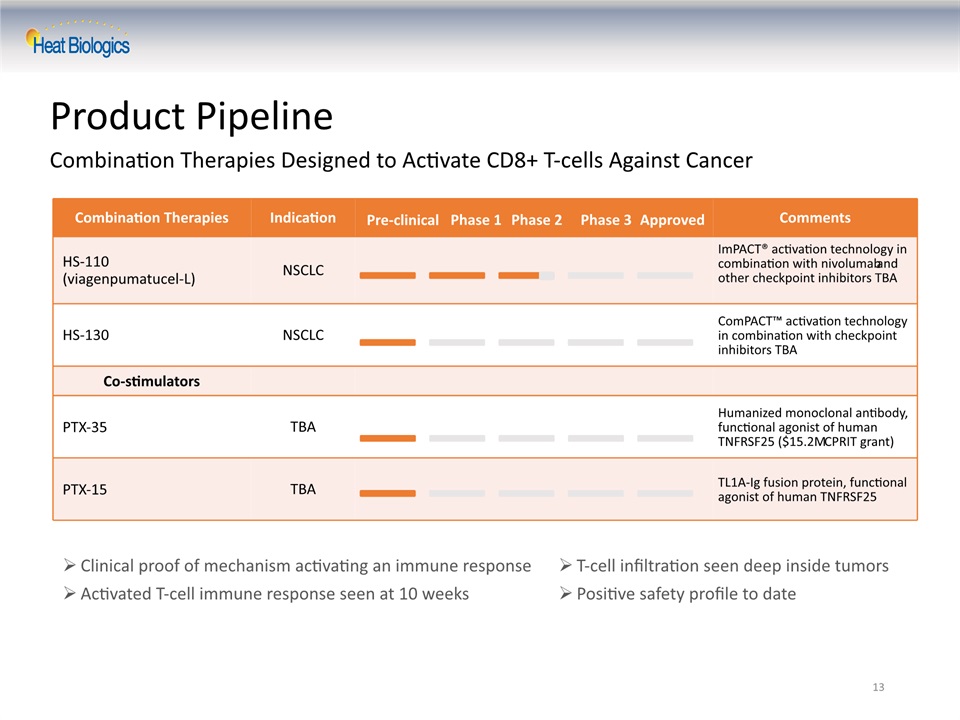
13 Product Pipeline Combination Therapies Designed to Activate CD8+ T-cells Against Cancer T-cell infiltration seen deep inside tumorsPositive safety profile to date Clinical proof of mechanism activating an immune responseActivated T-cell immune response seen at 10 weeks Combination Therapies Indication Comments HS-110(viagenpumatucel-L) NSCLC ImPACT® activation technology in combination with nivolumab and other checkpoint inhibitors TBA HS-130 NSCLC ComPACT™ activation technology in combination with checkpoint inhibitors TBA Co-stimulators PTX-35 TBA Humanized monoclonal antibody, functional agonist of human TNFRSF25 ($15.2M CPRIT grant) PTX-15 TBA TL1A-Ig fusion protein, functional agonist of human TNFRSF25 Pre-clinical Phase 1 Phase 2 Phase 3 Approved
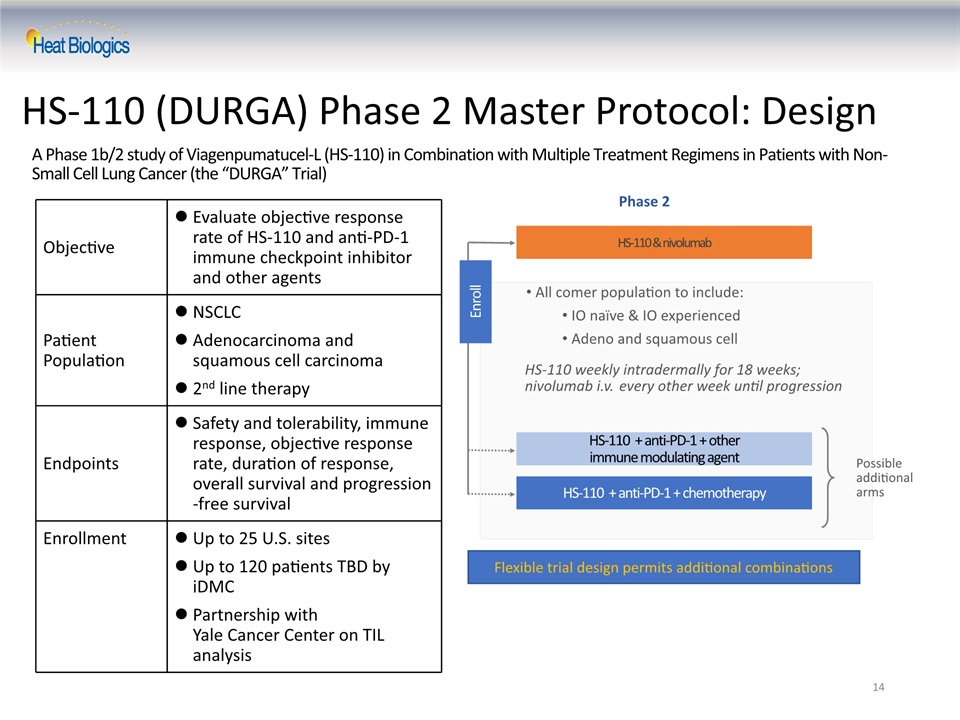
A Phase 1b/2 study of Viagenpumatucel-L (HS-110) in Combination with Multiple Treatment Regimens in Patients with Non-Small Cell Lung Cancer (the “DURGA” Trial) Objective Evaluate objective response rate of HS-110 and anti-PD-1 immune checkpoint inhibitor and other agents Patient Population NSCLCAdenocarcinoma and squamous cell carcinoma2nd line therapy Endpoints Safety and tolerability, immune response, objective response rate, duration of response, overall survival and progression-free survival Enrollment Up to 25 U.S. sitesUp to 120 patients TBD by iDMCPartnership with Yale Cancer Center on TIL analysis 14 HS-110 (DURGA) Phase 2 Master Protocol: Design HS-110 weekly intradermally for 18 weeks;nivolumab i.v. every other week until progression HS-110 + anti-PD-1 + other immune modulating agent HS-110 + anti-PD-1 + chemotherapy Phase 2 Possible additional arms All comer population to include:IO naïve & IO experiencedAdeno and squamous cell HS-110 & nivolumab Enroll Flexible trial design permits additional combinations
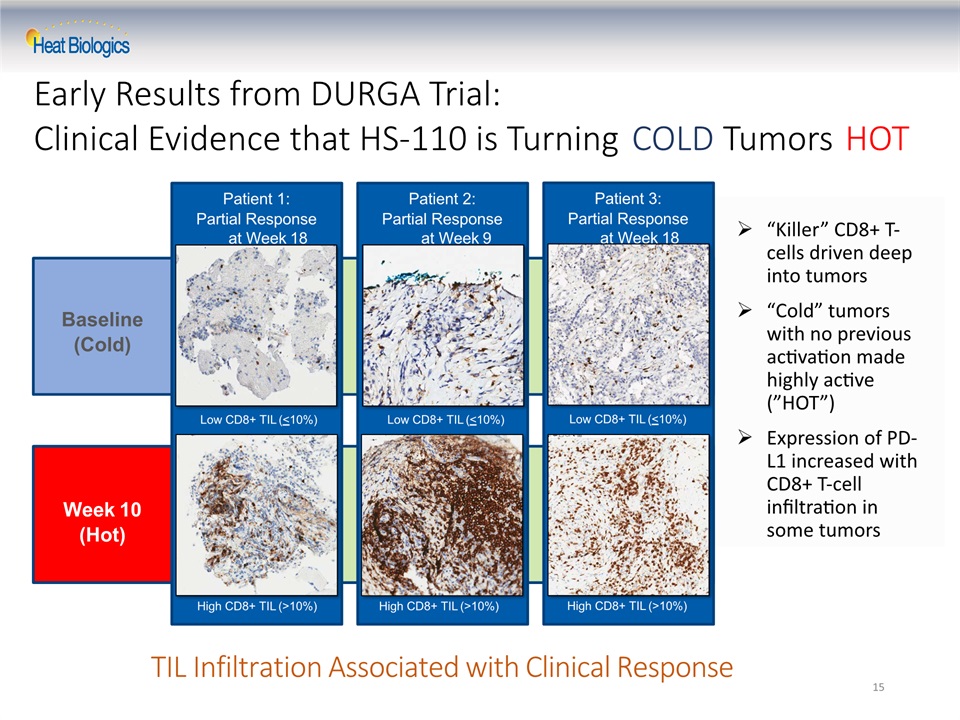
Baseline(Cold) Week 10(Hot) TIL Infiltration Associated with Clinical Response 15 Patient 1:Partial Response at Week 18 High CD8+ TIL (>10%) Patient 2:Partial Response at Week 9 High CD8+ TIL (>10%) Patient 3:Partial Response at Week 18 Low CD8+ TIL (<10%) High CD8+ TIL (>10%) “Killer” CD8+ T-cells driven deep into tumors“Cold” tumors with no previous activation made highly active (”HOT”)Expression of PD-L1 increased with CD8+ T-cell infiltration in some tumors Low CD8+ TIL (<10%) Low CD8+ TIL (<10%) Early Results from DURGA Trial: Clinical Evidence that HS-110 is Turning COLD Tumors HOT
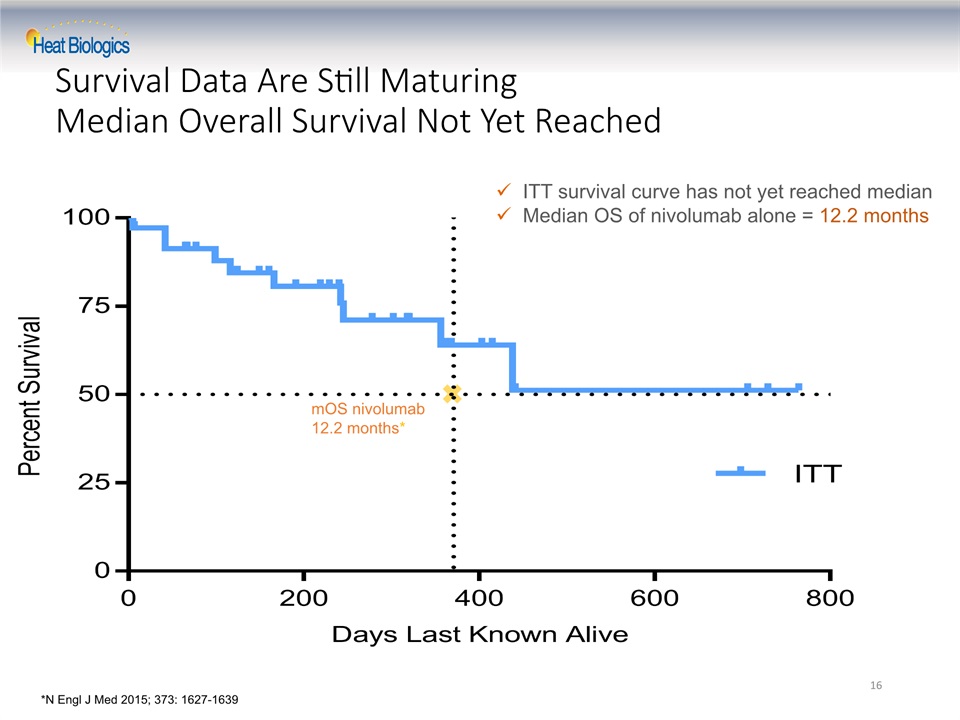
Survival Data Are Still MaturingMedian Overall Survival Not Yet Reached 16 ITT survival curve has not yet reached medianMedian OS of nivolumab alone = 12.2 months mOS nivolumab12.2 months* *N Engl J Med 2015; 373: 1627-1639
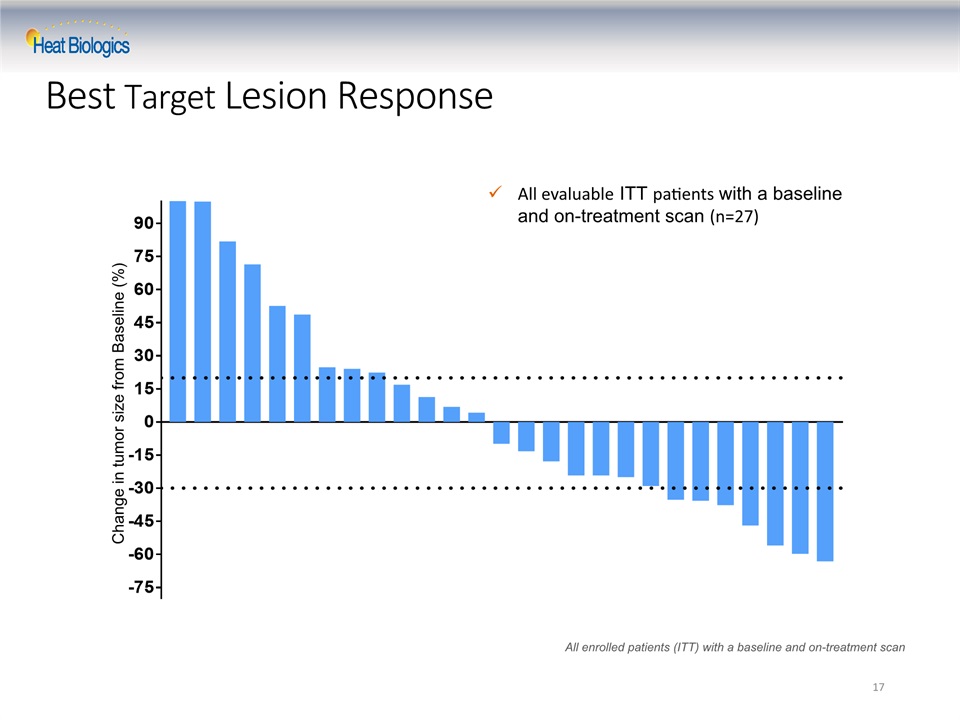
17 Change in tumor size from Baseline (%) All evaluable ITT patients with a baseline and on-treatment scan (n=27) All enrolled patients (ITT) with a baseline and on-treatment scan Best Target Lesion Response
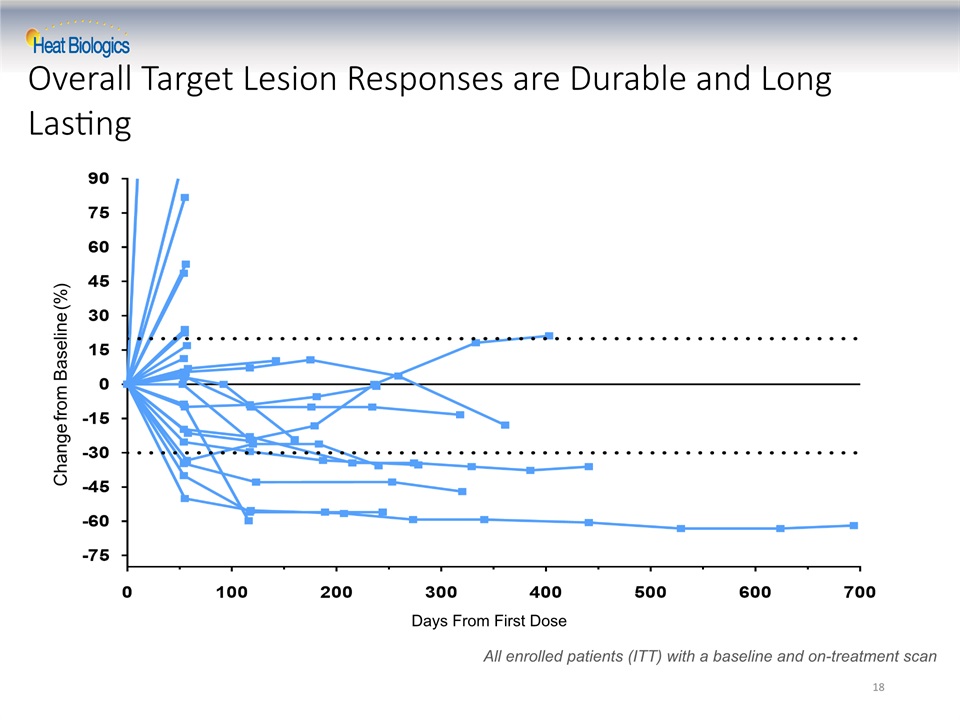
Overall Target Lesion Responses are Durable and Long Lasting Change from Baseline (%) All enrolled patients (ITT) with a baseline and on-treatment scan Days From First Dose 18
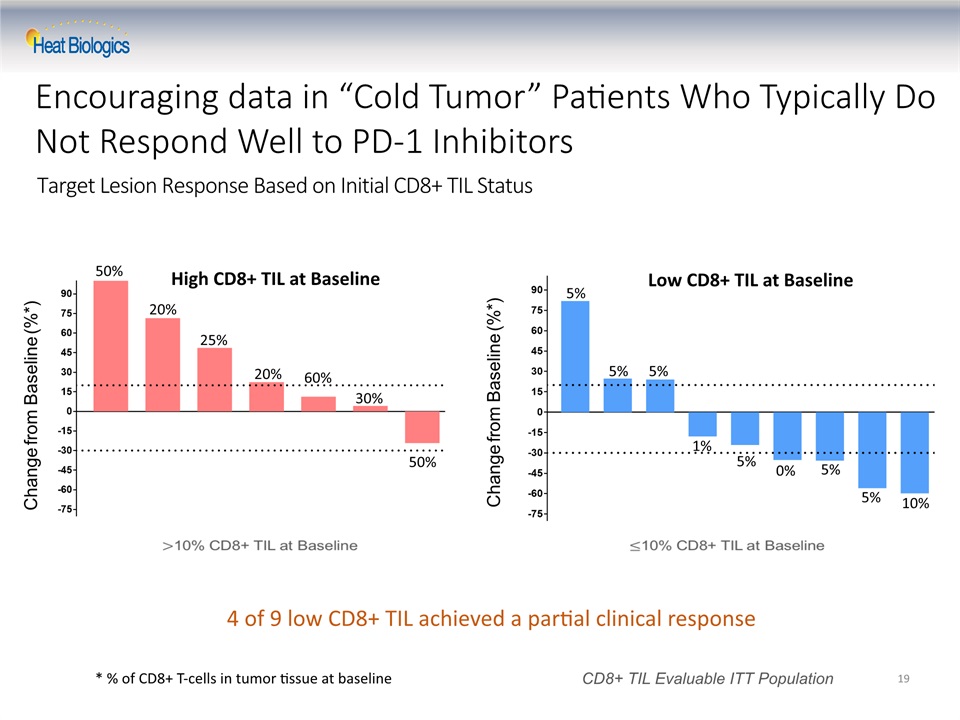
Target Lesion Response Based on Initial CD8+ TIL Status 19 Change from Baseline (%*) CD8+ TIL Evaluable ITT Population Change from Baseline (%*) High CD8+ TIL at Baseline Low CD8+ TIL at Baseline 50% 20% 25% 20% 60% 30% 50% 5% 5% 5% 5% 5% 5% 10% 0% 1% * % of CD8+ T-cells in tumor tissue at baseline Encouraging data in “Cold Tumor” Patients Who Typically Do Not Respond Well to PD-1 Inhibitors 4 of 9 low CD8+ TIL achieved a partial clinical response
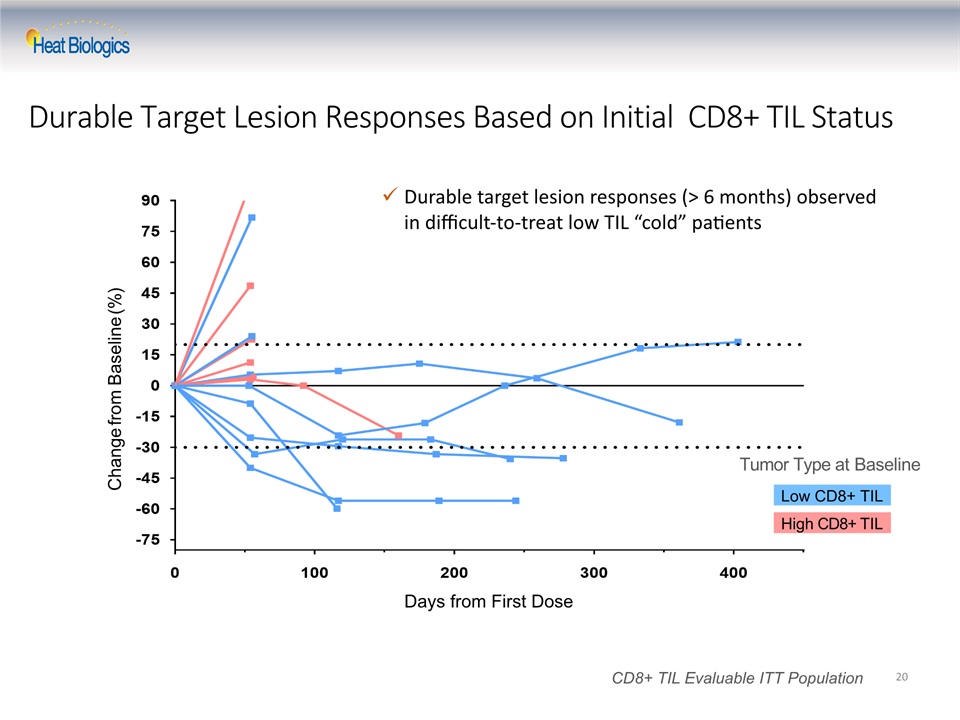
Durable Target Lesion Responses Based on Initial CD8+ TIL Status 20 Low CD8+ TIL High CD8+ TIL Change from Baseline (%) Tumor Type at Baseline CD8+ TIL Evaluable ITT Population Days from First Dose Durable target lesion responses (> 6 months) observed in difficult-to-treat low TIL “cold” patients
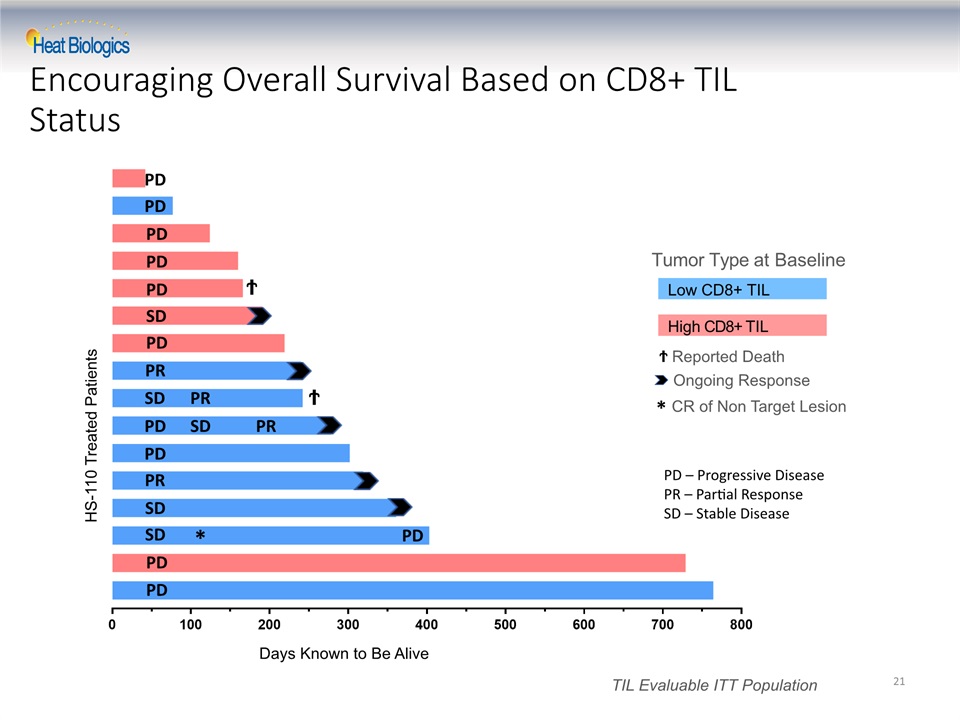
Encouraging Overall Survival Based on CD8+ TIL Status 21 HS-110 Treated Patients Days Known to Be Alive Low CD8+ TIL High CD8+ TIL Tumor Type at Baseline Ϯ Reported Death TIL Evaluable ITT Population Ϯ Ϯ PD PD PD PD PD PD PD PD PD PD SD SD SD SD SD PR PR PR PR PD * Ongoing Response CR of Non Target Lesion * PD – Progressive DiseasePR – Partial ResponseSD – Stable Disease
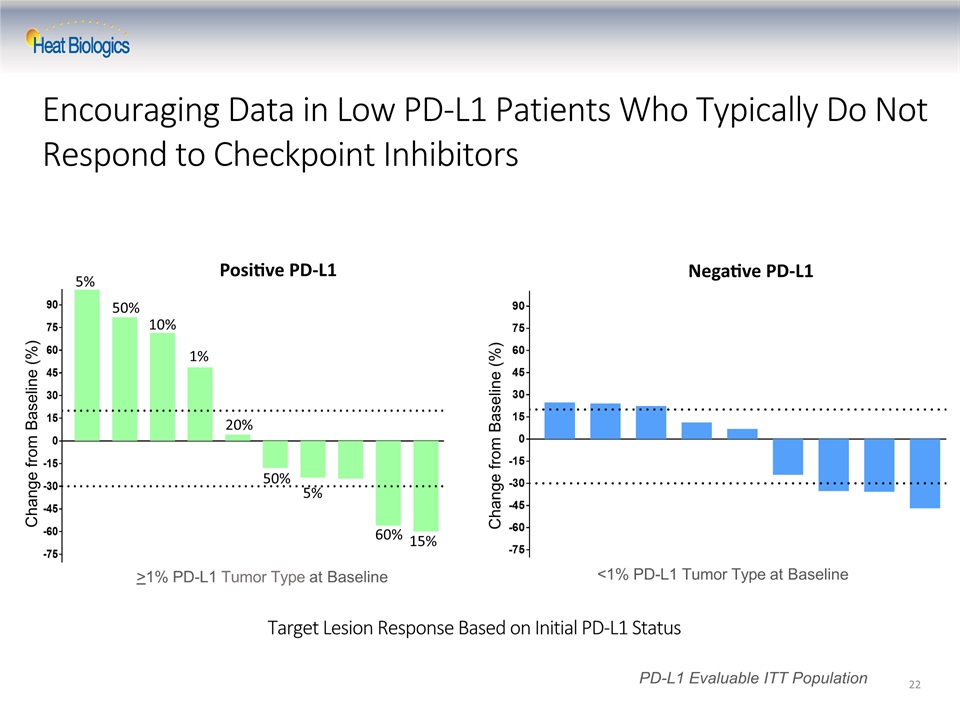
Target Lesion Response Based on Initial PD-L1 Status 22 Change from Baseline (%) PD-L1 Evaluable ITT Population Change from Baseline (%) >1% PD-L1 Tumor Type at Baseline <1% PD-L1 Tumor Type at Baseline Positive PD-L1 Negative PD-L1 5% 50% 10% 1% 20% 50% 5% 60% 15% Encouraging Data in Low PD-L1 Patients Who Typically Do Not Respond to Checkpoint Inhibitors
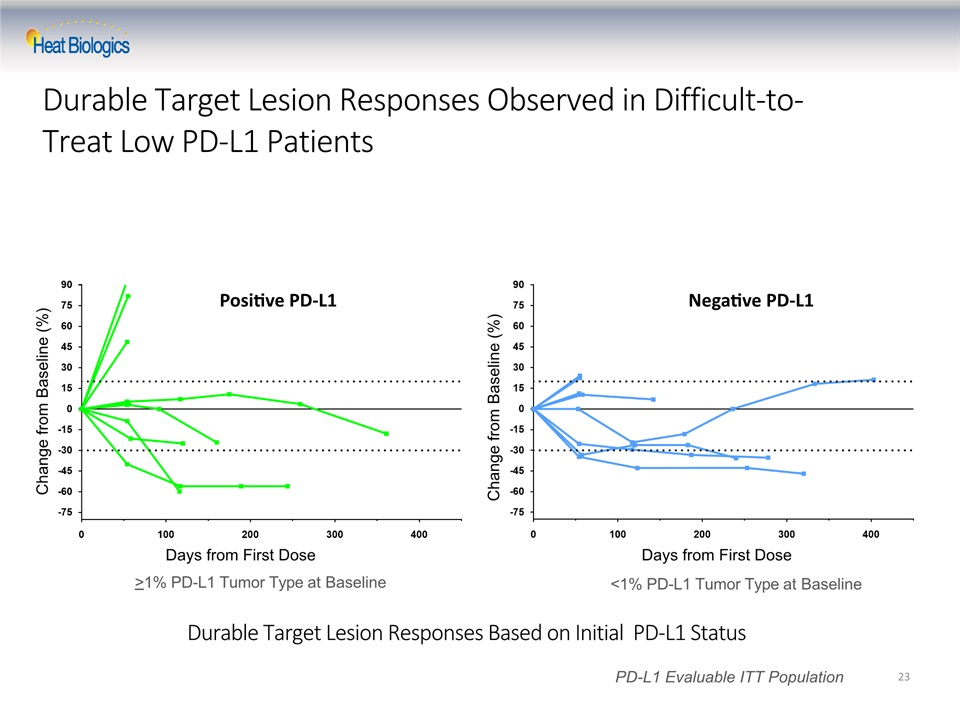
Durable Target Lesion Responses Based on Initial PD-L1 Status 23 Change from Baseline (%) PD-L1 Evaluable ITT Population Days from First Dose Change from Baseline (%) Days from First Dose >1% PD-L1 Tumor Type at Baseline <1% PD-L1 Tumor Type at Baseline Positive PD-L1 Negative PD-L1 Durable Target Lesion Responses Observed in Difficult-to-Treat Low PD-L1 Patients
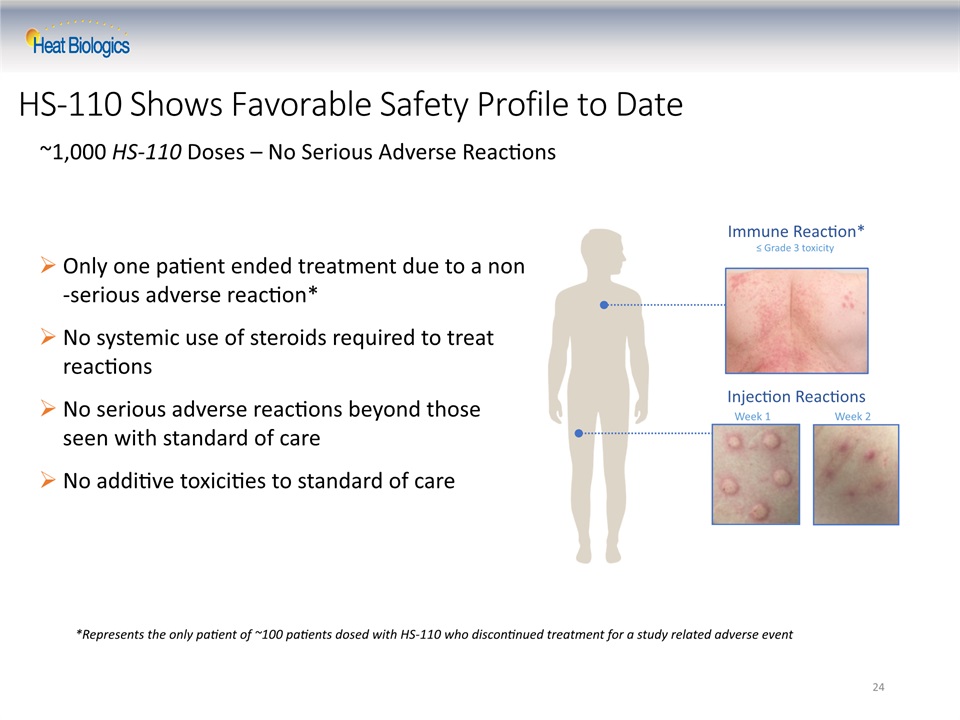
24 HS-110 Shows Favorable Safety Profile to Date ~1,000 HS-110 Doses – No Serious Adverse Reactions Only one patient ended treatment due to a non-serious adverse reaction*No systemic use of steroids required to treat reactionsNo serious adverse reactions beyond those seen with standard of careNo additive toxicities to standard of care *Represents the only patient of ~100 patients dosed with HS-110 who discontinued treatment for a study related adverse event Immune Reaction*≤ Grade 3 toxicity Week 1 Week 2 Injection Reactions

Summary of Phase 2 Interim Data Tumor shrinkage and disease control demonstrated in a majority of evaluable patientsOverall responses are durable and long lastingMedian overall survival has not yet been reachedHS-110 shows effect in difficult-to-treat low TIL and low PD-L1 patients who typically do not respond to checkpoint inhibitorsHS-110 shows durable responses in low TIL “cold tumor” patientsHS-110 shows durable responses in low PD-L1 patientsA trend of survival benefit is observed with higher ELISPOT activity reflective of tumor antigen-specific immune response 25
26 Heat Biologics Acquires Pelican Therapeutics Unlocking the Body’s Natural Defenses with a Broad Range of Combination Therapies Heat acquired 80% controlling interest in Pelican in May 2017Pre-clinical synergy with Heat’s ImPACT® and checkpoint therapy$15.2M grant award from the Cancer Prevention Research Institute of Texas (CPRIT) propels PTX-35 through a 70-patient, first-in-human clinical programPTX-35 is a potential best-in-class, T-cell co-stimulator specific to “killer” CD8+ “memory” T-cells Pre-clinical studies highlight advantages over competing T-cell co-stimulator programs based on CD8+ T-cell specificity Only Pelican is targeting TNFRSF25, an emerging target in immuno-oncology
27 Highlights from Pelican Pre-clinical Studies TNFRSF25 is preferentially expressed on CD8+ T-cells compared to other T-cell co-stimulatorsCo-stimulation occurs only in the context of TCR recognition of antigenDrives the development of antigen-specific CD8+ T-cells (mimics TL1A, the specific ligand of TNFRSF25)Superior activity is seen with TNFRSF25 in stimulating memory CD8+ cells relative to OX40, 4-1BB and GITRAgonism with TNFRSF25 mAb increases:Effector cytokine functionEffector immune functionSurvival in mouse modelsTNFRSF25 mAb results in increased survival compared to agonism of OX40, GITR, 4-1BB with respective agonist mAbs in mouse melanoma models
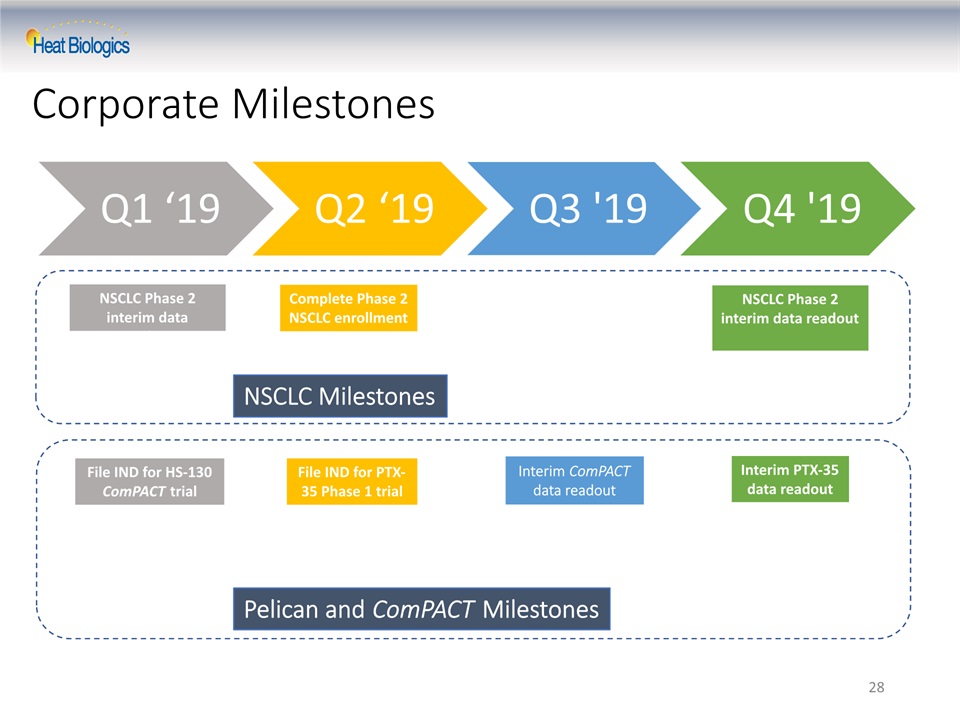
28 NSCLC Phase 2 interim data readout File IND for HS-130 ComPACT trial File IND for PTX-35 Phase 1 trial Complete Phase 2 NSCLC enrollment NSCLC Milestones Pelican and ComPACT Milestones Interim PTX-35 data readout Interim ComPACT data readout NSCLC Phase 2 interim data readout Corporate Milestones
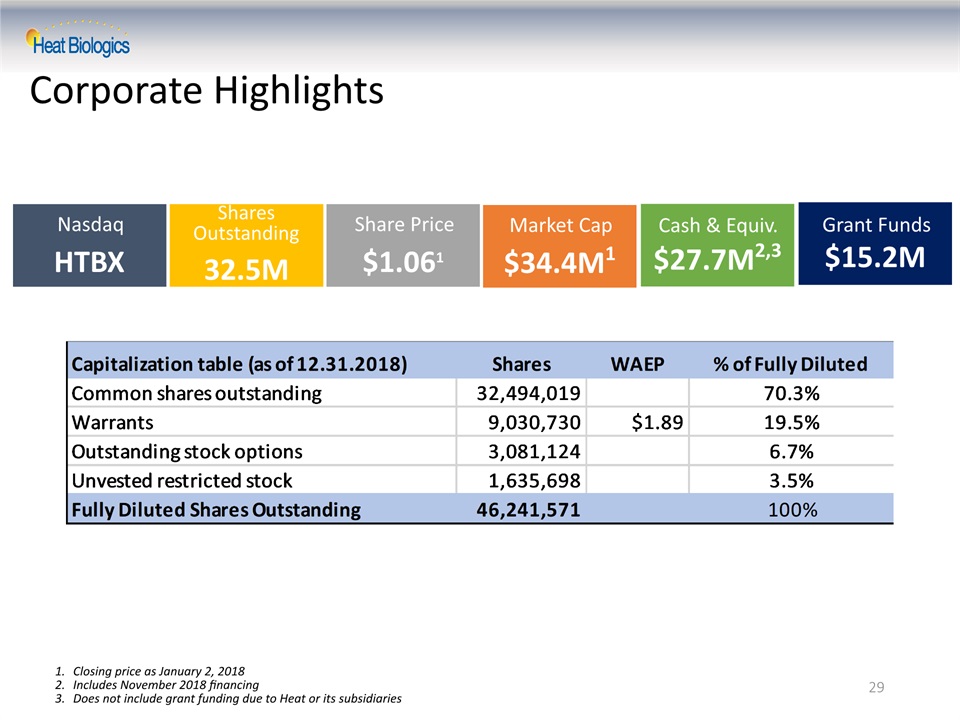
29 Market Cap$34.4M1 Cash & Equiv.$27.7M2,3 NasdaqHTBX Shares Outstanding32.5M Share Price$1.061 Grant Funds$15.2M Corporate Highlights Closing price as January 2, 2018Includes November 2018 financingDoes not include grant funding due to Heat or its subsidiaries

Investment Highlights Potential Best in Class Oncology Treatment Off-the-shelf Therapies Clinical Data with Checkpoint Inhibitors Diverse Technology Platforms Strong Balance Sheet 30
Cold Tumors Hot Tumors Turning COLD Tumors HOT 31
Appendix 32
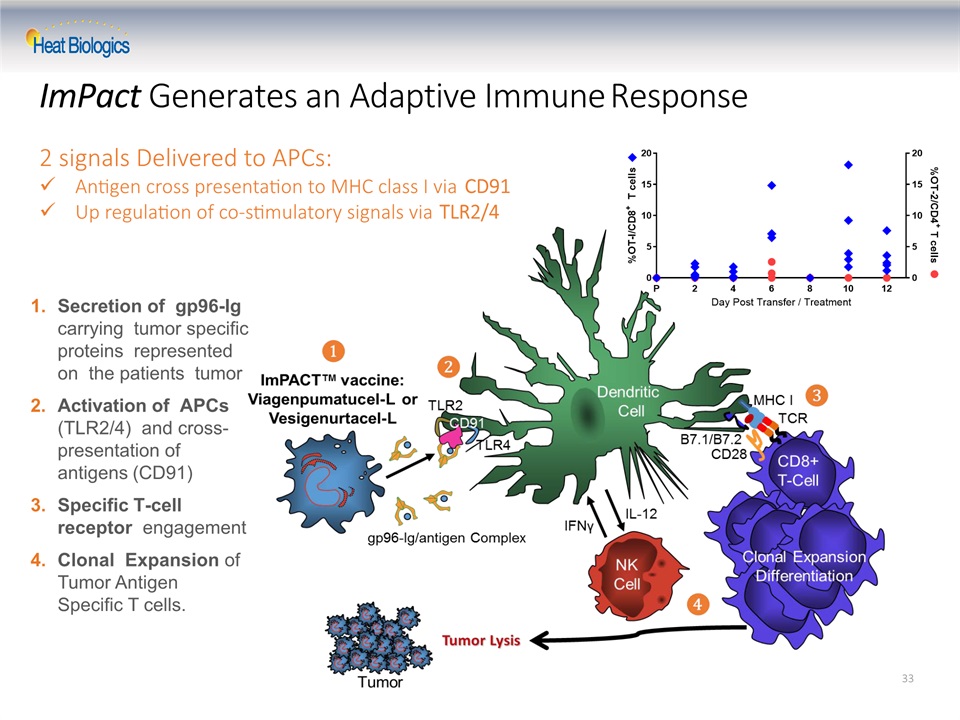
ImPact Generates an Adaptive Immune Response Secretion of gp96-Ig carrying tumor specific proteins represented on the patients tumorActivation of APCs (TLR2/4) and cross- presentation of antigens (CD91)Specific T-cell receptor engagementClonal Expansion of Tumor Antigen Specific T cells. 33 2 signals Delivered to APCs: Antigen cross presentation to MHC class I via CD91Up regulation of co-stimulatory signals via TLR2/4
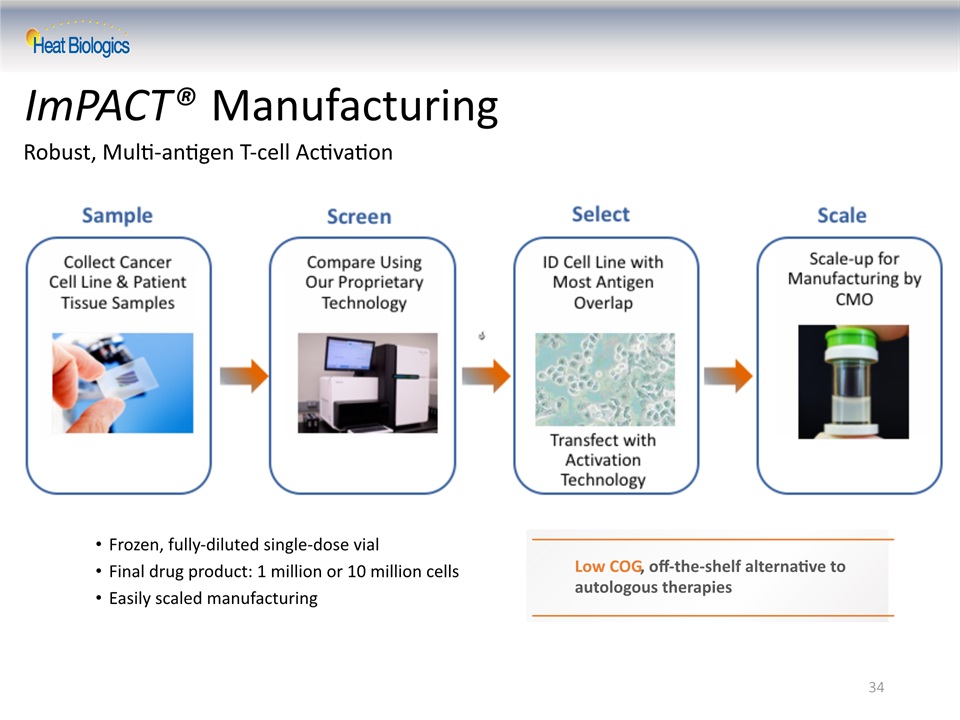
34 ImPACT® Manufacturing Robust, Multi-antigen T-cell Activation Frozen, fully-diluted single-dose vialFinal drug product: 1 million or 10 million cellsEasily scaled manufacturing Low COG, off-the-shelf alternative to autologous therapies
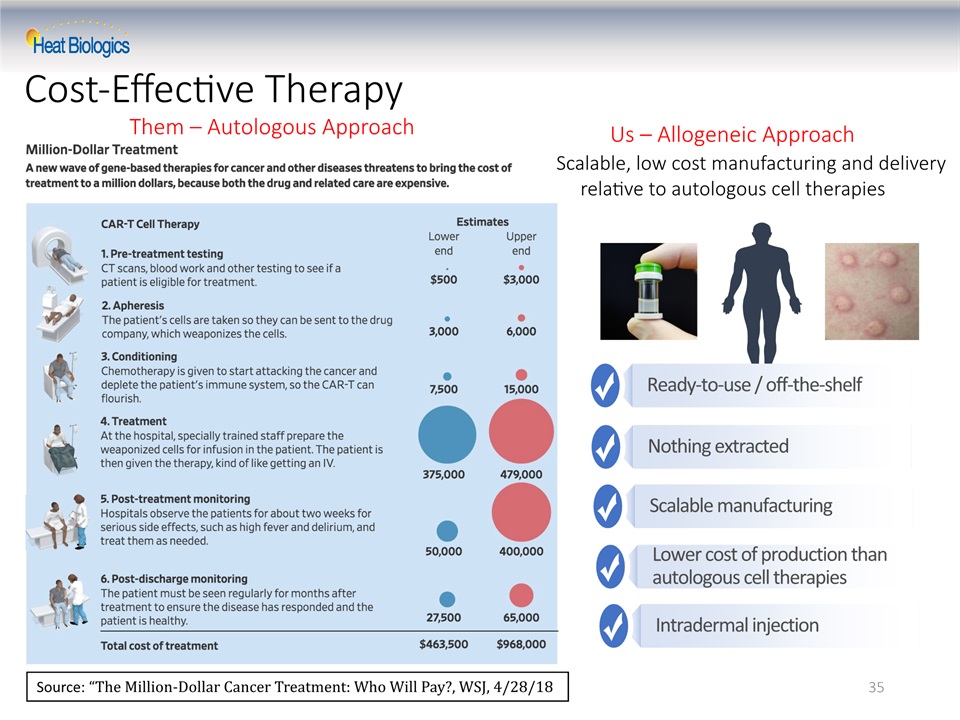
Cost-Effective Therapy 35 Source: “The Million-Dollar Cancer Treatment: Who Will Pay?, WSJ, 4/28/18 Them – Autologous Approach Us – Allogeneic Approach Scalable manufacturing Nothing extracted Ready-to-use / off-the-shelf Intradermal injection Lower cost of production than autologous cell therapies Scalable, low cost manufacturing and delivery relative to autologous cell therapies
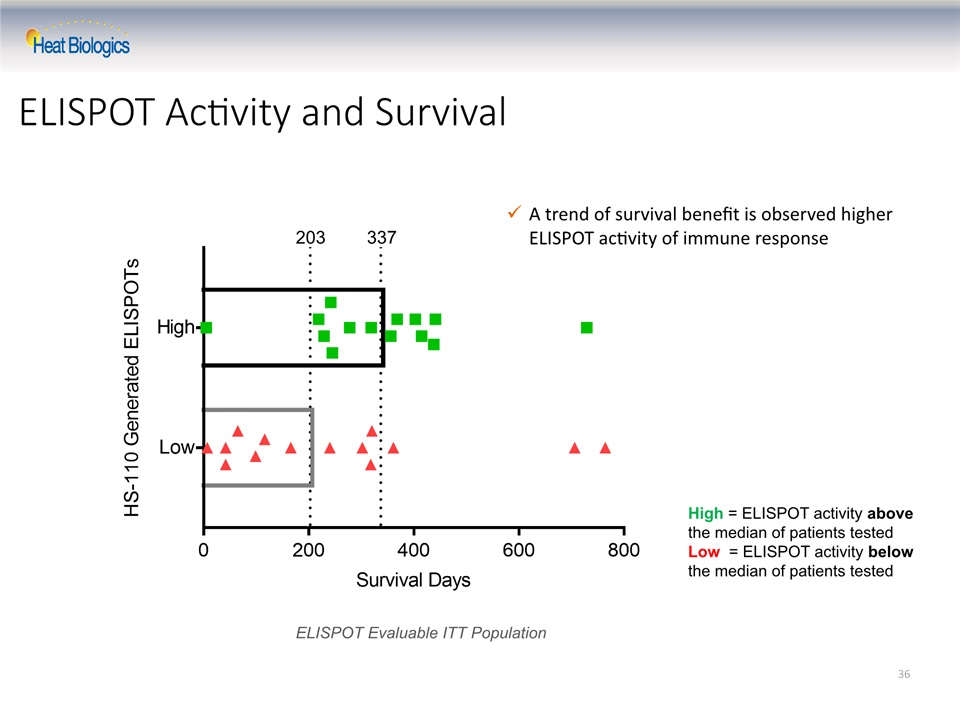
ELISPOT Activity and Survival 36 High = ELISPOT activity above the median of patients tested Low = ELISPOT activity below the median of patients tested A trend of survival benefit is observed higher ELISPOT activity of immune response 203 337 ELISPOT Evaluable ITT Population
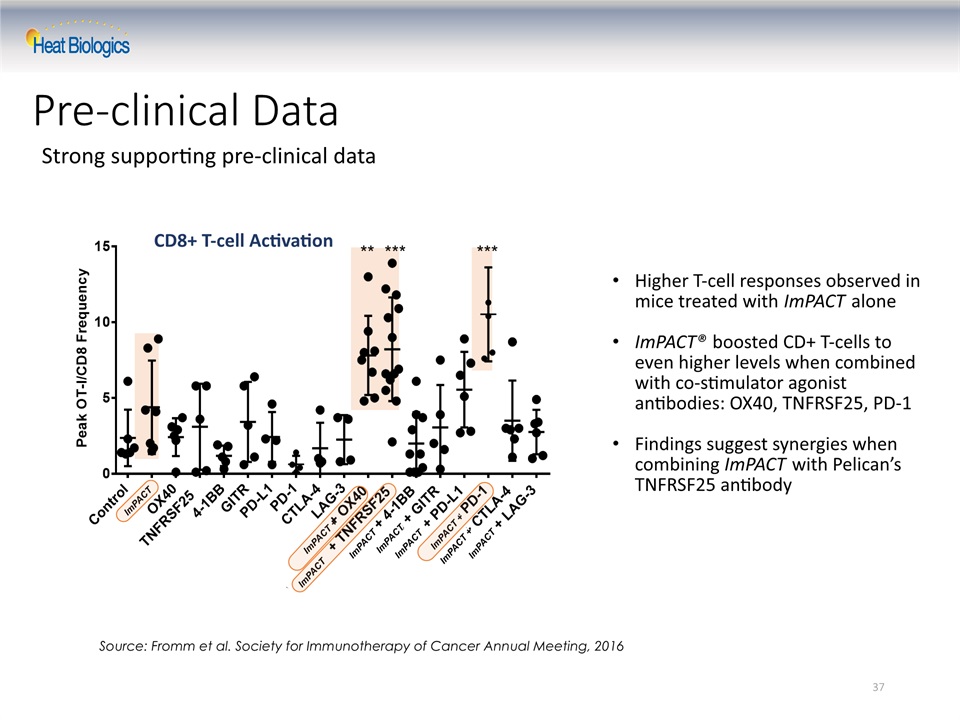
Strong supporting pre-clinical data 37 Pre-clinical Data Source: Fromm et al. Society for Immunotherapy of Cancer Annual Meeting, 2016 CD8+ T-cell Activation Higher T-cell responses observed in mice treated with ImPACT aloneImPACT® boosted CD+ T-cells to even higher levels when combined with co-stimulator agonist antibodies: OX40, TNFRSF25, PD-1Findings suggest synergies when combining ImPACT with Pelican’s TNFRSF25 antibody ImPACT + ImPACT ImPACT ImPACT ImPACT + ImPACT ImPACT ImPACT ImPACT +

38 ComPACT™ Platform Technology gp96-Ig OX40L-Fc The first potential dual-acting immunotherapy designed to deliver T-cell activation andco-stimulation in a single product – combination therapy without additive costs
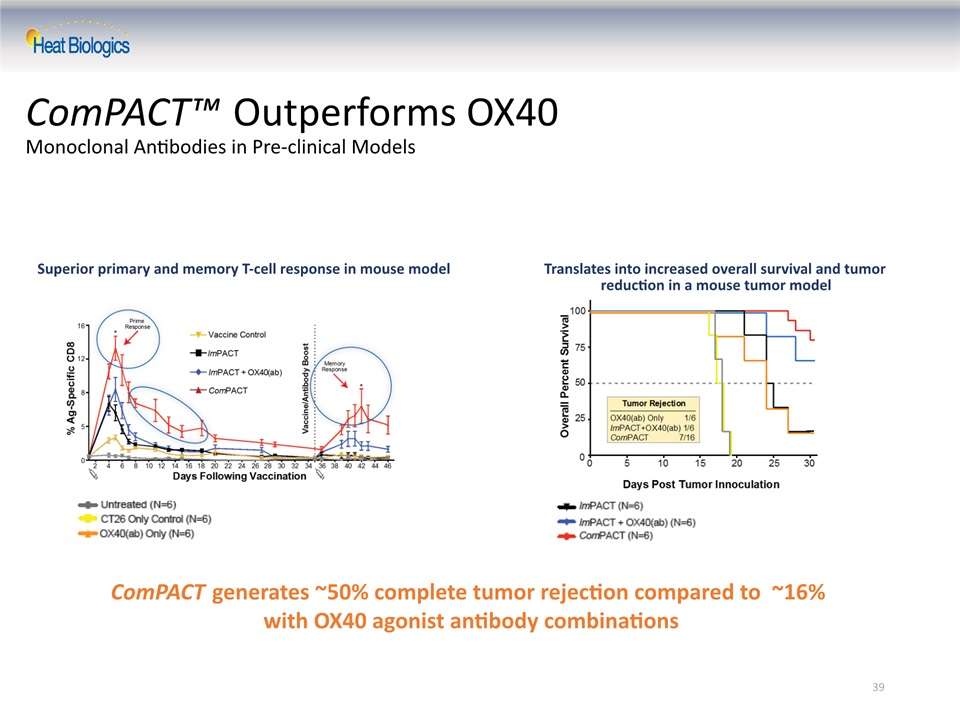
39 ComPACT™ Outperforms OX40 Monoclonal Antibodies in Pre-clinical Models ComPACT generates ~50% complete tumor rejection compared to ~16% with OX40 agonist antibody combinations Superior primary and memory T-cell response in mouse model Translates into increased overall survival and tumor reduction in a mouse tumor model
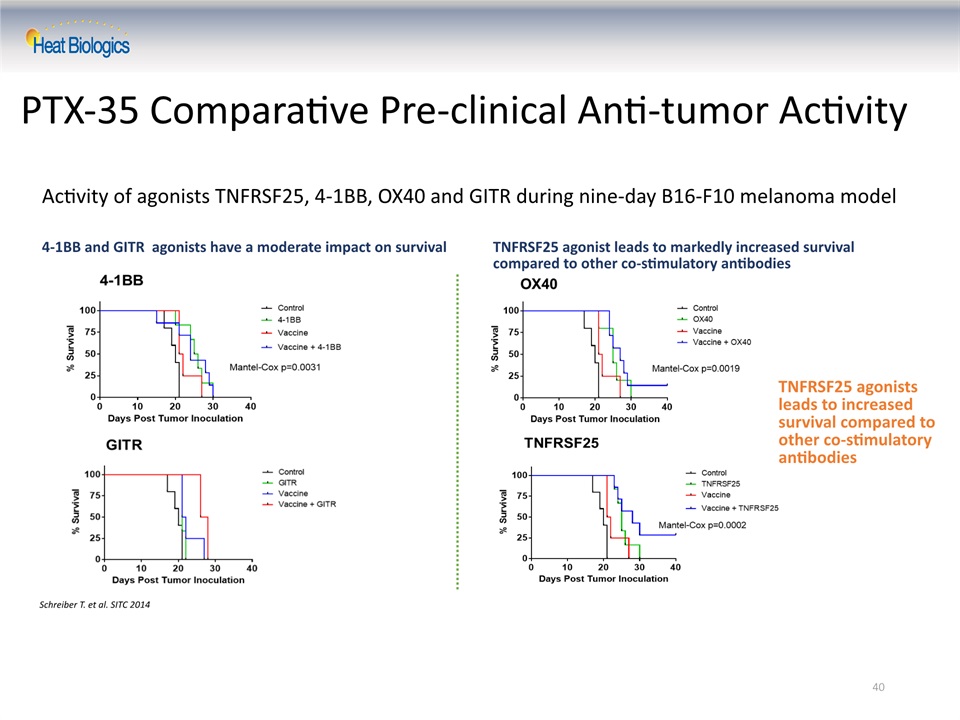
40 PTX-35 Comparative Pre-clinical Anti-tumor Activity Schreiber T. et al. SITC 2014 Activity of agonists TNFRSF25, 4-1BB, OX40 and GITR during nine-day B16-F10 melanoma model TNFRSF25 agonists leads to increased survival compared to other co-stimulatory antibodies 4-1BB and GITR agonists have a moderate impact on survival TNFRSF25 agonist leads to markedly increased survival compared to other co-stimulatory antibodies
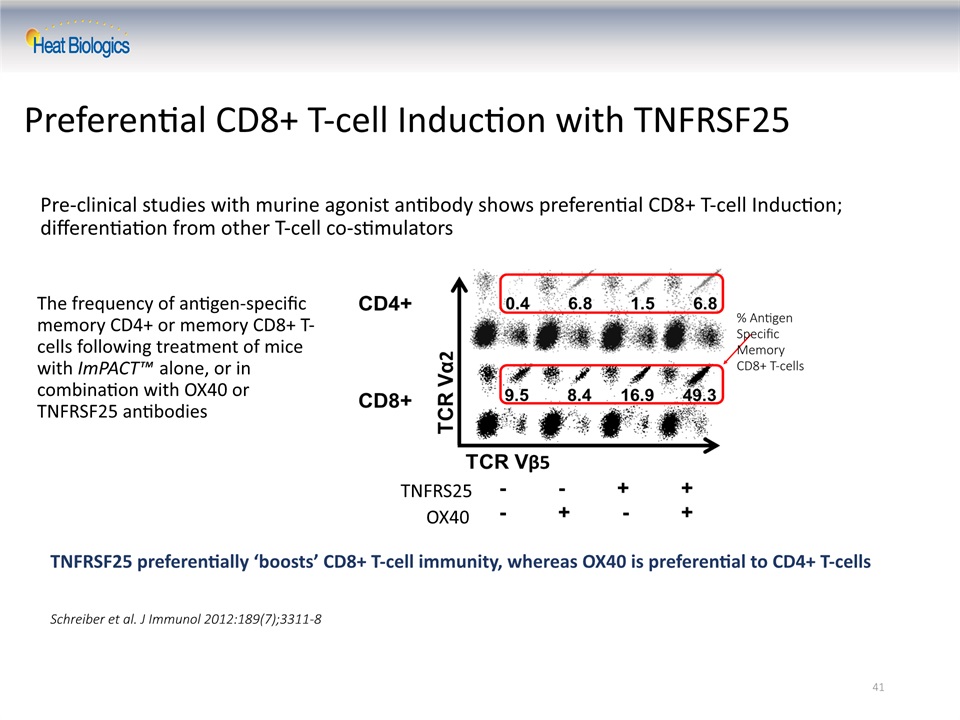
41 Preferential CD8+ T-cell Induction with TNFRSF25 Pre-clinical studies with murine agonist antibody shows preferential CD8+ T-cell Induction; differentiation from other T-cell co-stimulators The frequency of antigen-specific memory CD4+ or memory CD8+ T-cells following treatment of mice with ImPACT™ alone, or in combination with OX40 or TNFRSF25 antibodies Schreiber et al. J Immunol 2012:189(7);3311-8 % AntigenSpecific Memory CD8+ T-cells TNFRS25 OX40 TNFRSF25 preferentially ‘boosts’ CD8+ T-cell immunity, whereas OX40 is preferential to CD4+ T-cells
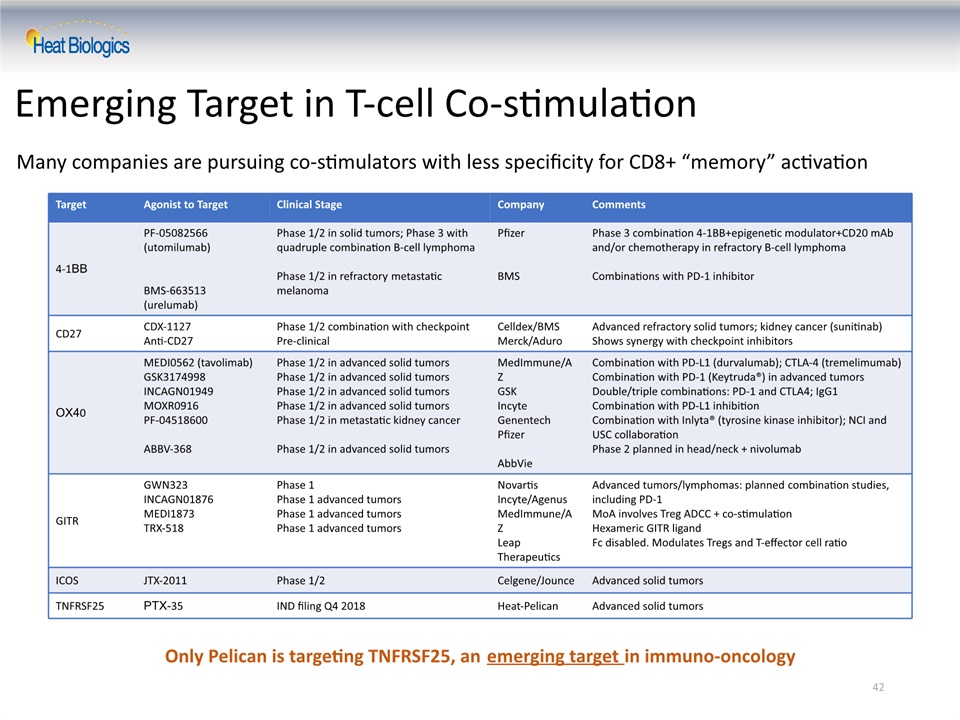
Many companies are pursuing co-stimulators with less specificity for CD8+ “memory” activation 42 Emerging Target in T-cell Co-stimulation Only Pelican is targeting TNFRSF25, an emerging target in immuno-oncology Target Agonist to Target Clinical Stage Company Comments 4-1BB PF-05082566 (utomilumab)BMS-663513 (urelumab) Phase 1/2 in solid tumors; Phase 3 with quadruple combination B-cell lymphomaPhase 1/2 in refractory metastatic melanoma PfizerBMS Phase 3 combination 4-1BB+epigenetic modulator+CD20 mAb and/or chemotherapy in refractory B-cell lymphomaCombinations with PD-1 inhibitor CD27 CDX-1127Anti-CD27 Phase 1/2 combination with checkpointPre-clinical Celldex/BMSMerck/Aduro Advanced refractory solid tumors; kidney cancer (sunitinab)Shows synergy with checkpoint inhibitors OX40 MEDI0562 (tavolimab)GSK3174998INCAGN01949MOXR0916PF-04518600ABBV-368 Phase 1/2 in advanced solid tumorsPhase 1/2 in advanced solid tumorsPhase 1/2 in advanced solid tumorsPhase 1/2 in advanced solid tumorsPhase 1/2 in metastatic kidney cancerPhase 1/2 in advanced solid tumors MedImmune/AZGSKIncyteGenentechPfizerAbbVie Combination with PD-L1 (durvalumab); CTLA-4 (tremelimumab)Combination with PD-1 (Keytruda®) in advanced tumorsDouble/triple combinations: PD-1 and CTLA4; IgG1Combination with PD-L1 inhibitionCombination with Inlyta® (tyrosine kinase inhibitor); NCI and USC collaborationPhase 2 planned in head/neck + nivolumab GITR GWN323INCAGN01876MEDI1873TRX-518 Phase 1Phase 1 advanced tumorsPhase 1 advanced tumorsPhase 1 advanced tumors NovartisIncyte/AgenusMedImmune/AZLeap Therapeutics Advanced tumors/lymphomas: planned combination studies, including PD-1MoA involves Treg ADCC + co-stimulationHexameric GITR ligandFc disabled. Modulates Tregs and T-effector cell ratio ICOS JTX-2011 Phase 1/2 Celgene/Jounce Advanced solid tumors TNFRSF25 PTX-35 IND filing Q4 2018 Heat-Pelican Advanced solid tumors
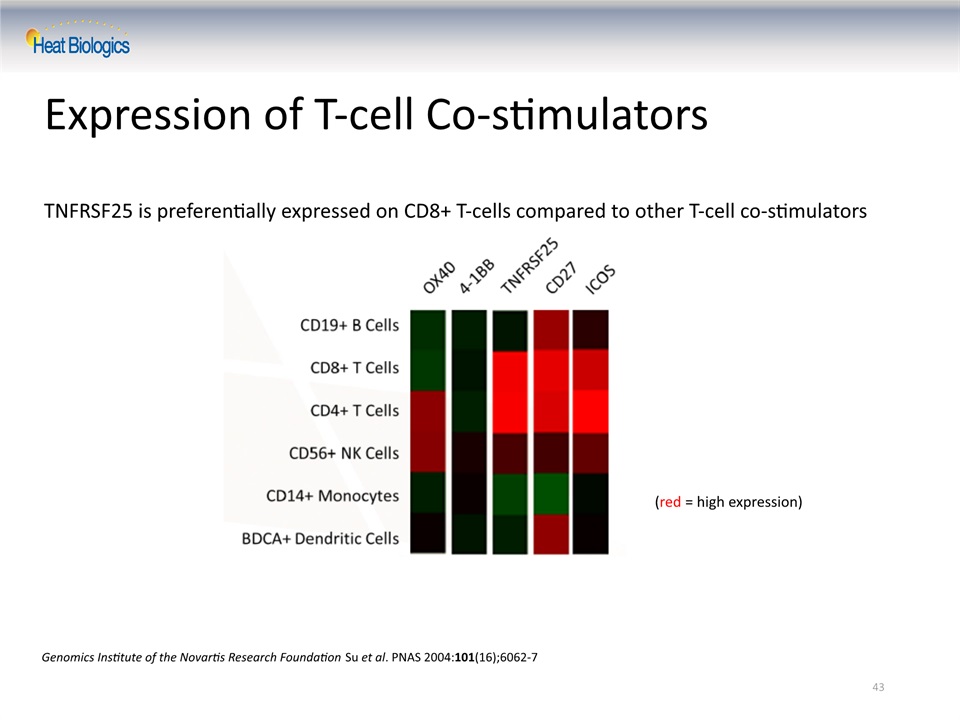
43 Expression of T-cell Co-stimulators TNFRSF25 is preferentially expressed on CD8+ T-cells compared to other T-cell co-stimulators Genomics Institute of the Novartis Research Foundation Su et al. PNAS 2004:101(16);6062-7 (red = high expression)