POSTER
Published on February 28, 2019
EXHIBIT 99.1
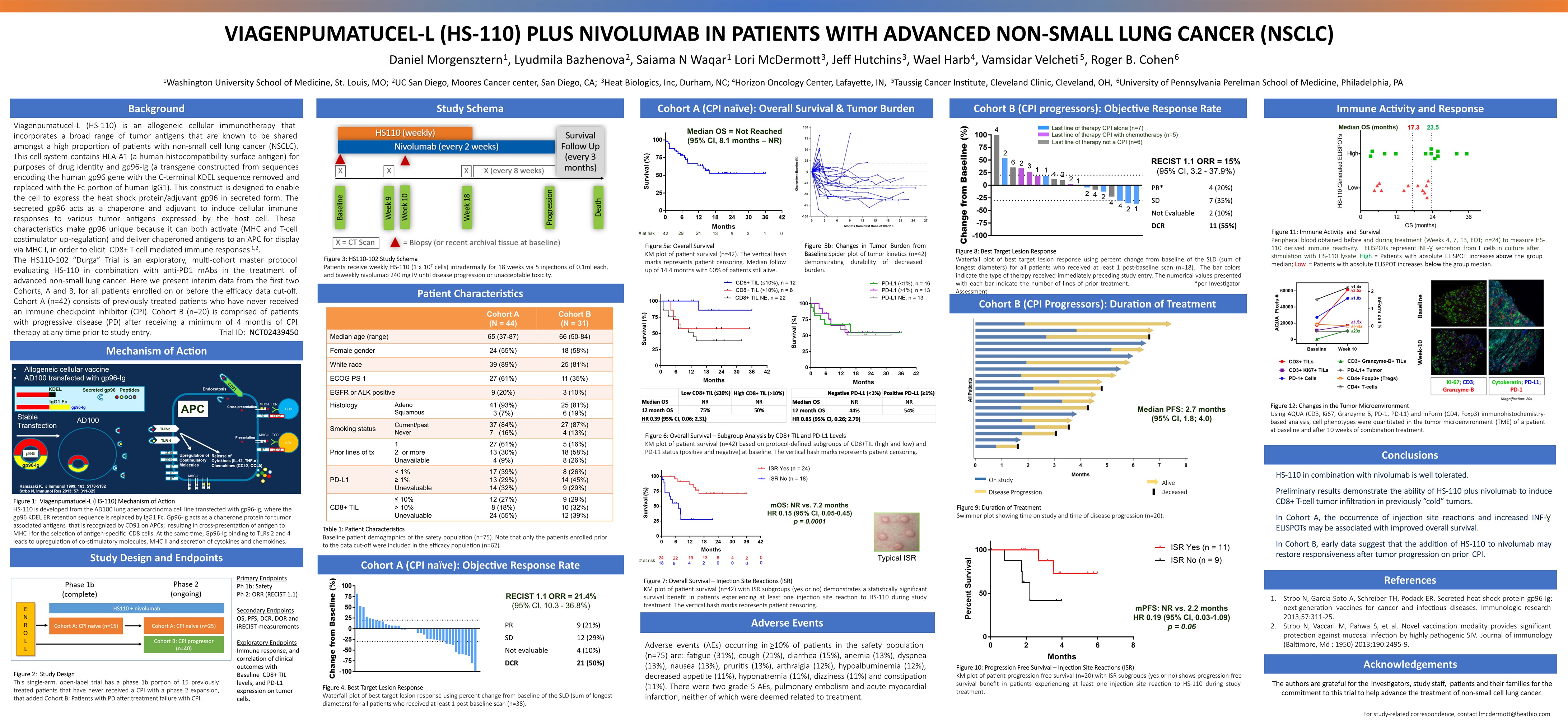
VIAGENPUMATUCEL-L (HS-110) PLUS NIVOLUMAB IN PATIENTS WITH ADVANCED NON-SMALL LUNG CANCER (NSCLC) Daniel Morgensztern1, Lyudmila Bazhenova2, Saiama N Waqar1 Lori McDermott3, Jeff Hutchins3, Wael Harb4, Vamsidar Velcheti5, Roger B. Cohen61Washington University School of Medicine, St. Louis, MO; 2UC San Diego, Moores Cancer center, San Diego, CA; 3Heat Biologics, Inc, Durham, NC; 4Horizon Oncology Center, Lafayette, IN, 5Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, 6University of Pennsylvania Perelman School of Medicine, Philadelphia, PA Background Acknowledgements The authors are grateful for the Investigators, study staff, patients and their families for the commitment to this trial to help advance the treatment of non-small cell lung cancer. Study Schema Patient Characteristics Cohort B (CPI progressors): Objective Response Rate Viagenpumatucel-L (HS-110) is an allogeneic cellular immunotherapy that incorporates a broad range of tumor antigens that are known to be shared amongst a high proportion of patients with non-small cell lung cancer (NSCLC). This cell system contains HLA-A1 (a human histocompatibility surface antigen) for purposes of drug identity and gp96-Ig (a transgene constructed from sequences encoding the human gp96 gene with the C-terminal KDEL sequence removed and replaced with the Fc portion of human IgG1). This construct is designed to enable the cell to express the heat shock protein/adjuvant gp96 in secreted form. The secreted gp96 acts as a chaperone and adjuvant to induce cellular immune responses to various tumor antigens expressed by the host cell. These characteristics make gp96 unique because it can both activate (MHC and T-cell costimulator up-regulation) and deliver chaperoned antigens to an APC for display via MHC I, in order to elicit CD8+ T-cell mediated immune responses1,2.The HS110-102 “Durga” Trial is an exploratory, multi-cohort master protocol evaluating HS-110 in combination with anti-PD1 mAbs in the treatment of advanced non-small lung cancer. Here we present interim data from the first two Cohorts, A and B, for all patients enrolled on or before the efficacy data cut-off. Cohort A (n=42) consists of previously treated patients who have never received an immune checkpoint inhibitor (CPI). Cohort B (n=20) is comprised of patients with progressive disease (PD) after receiving a minimum of 4 months of CPI therapy at any time prior to study entry. Trial ID: NCT02439450 References Strbo N, Garcia-Soto A, Schreiber TH, Podack ER. Secreted heat shock protein gp96-Ig: next-generation vaccines for cancer and infectious diseases. Immunologic research 2013;57:311-25. Strbo N, Vaccari M, Pahwa S, et al. Novel vaccination modality provides significant protection against mucosal infection by highly pathogenic SIV. Journal of immunology (Baltimore, Md : 1950) 2013;190:2495-9. Study Design and Endpoints Cohort A (CPI naïve): Overall Survival & Tumor Burden Conclusions Figure 3: HS110-102 Study SchemaPatients receive weekly HS-110 (1 x 107 cells) intradermally for 18 weeks via 5 injections of 0.1ml each, and biweekly nivolumab 240 mg IV until disease progression or unacceptable toxicity. Table 1: Patient CharacteristicsBaseline patient demographics of the safety population (n=75). Note that only the patients enrolled prior to the data cut-off were included in the efficacy population (n=62). Immune Activity and Response Mechanism of Action Figure 1: Viagenpumatucel-L (HS-110) Mechanism of ActionHS-110 is developed from the AD100 lung adenocarcinoma cell line transfected with gp96-Ig, where the gp96 KDEL ER retention sequence is replaced by IgG1 Fc. Gp96-Ig acts as a chaperone protein for tumor associated antigens that is recognized by CD91 on APCs; resulting in cross-presentation of antigen to MHC I for the selection of antigen-specific CD8 cells. At the same time, Gp96-Ig binding to TLRs 2 and 4 leads to upregulation of co-stimulatory molecules, MHC II and secretion of cytokines and chemokines. Cohort A (CPI naïve): Objective Response Rate Figure 4: Best Target Lesion ResponseWaterfall plot of best target lesion response using percent change from baseline of the SLD (sum of longest diameters) for all patients who received at least 1 post-baseline scan (n=38). HS-110 in combination with nivolumab is well tolerated. Preliminary results demonstrate the ability of HS-110 plus nivolumab to induce CD8+ T-cell tumor infiltration in previously “cold” tumors.In Cohort A, the occurrence of injection site reactions and increased INF-Ɣ ELISPOTs may be associated with improved overall survival. In Cohort B, early data suggest that the addition of HS-110 to nivolumab may restore responsiveness after tumor progression on prior CPI. Cohort A (N = 44) Cohort B (N = 31) Median age (range) 65 (37-87) 66 (50-84) Female gender 24 (55%) 18 (58%) White race 39 (89%) 25 (81%) ECOG PS 1 27 (61%) 11 (35%) EGFR or ALK positive 9 (20%) 3 (10%) Histology AdenoSquamous 41 (93%)3 (7%) 25 (81%)6 (19%) Smoking status Current/pastNever 37 (84%)7 (16%) 27 (87%)4 (13%) Prior lines of tx 12 or more Unavailable 27 (61%)13 (30%)4 (9%) 5 (16%)18 (58%)8 (26%) PD-L1 < 1%≥ 1%Unevaluable 17 (39%)13 (29%)14 (32%) 8 (26%)14 (45%)9 (29%) CD8+ TIL ≤ 10%> 10%Unevaluable 12 (27%)8 (18%)24 (55%) 9 (29%)10 (32%)12 (39%) PR 9 (21%) SD 12 (29%) Not evaluable 4 (10%) DCR 21 (50%) Cohort B (CPI Progressors): Duration of Treatment mOS: NR vs. 7.2 monthsHR 0.15 (95% CI, 0.05-0.45)p = 0.0001 Typical ISR Figure 5a: Overall Survival KM plot of patient survival (n=42). The vertical hash marks represents patient censoring. Median follow up of 14.4 months with 60% of patients still alive. Figure 6: Overall Survival – Subgroup Analysis by CD8+ TIL and PD-L1 LevelsKM plot of patient survival (n=42) based on protocol-defined subgroups of CD8+TIL (high and low) and PD-L1 status (positive and negative) at baseline. The vertical hash marks represents patient censoring. Figure 7: Overall Survival – Injection Site Reactions (ISR)KM plot of patient survival (n=42) with ISR subgroups (yes or no) demonstrates a statistically significant survival benefit in patients experiencing at least one injection site reaction to HS-110 during study treatment. The vertical hash marks represents patient censoring. Figure 8: Best Target Lesion ResponseWaterfall plot of best target lesion response using percent change from baseline of the SLD (sum of longest diameters) for all patients who received at least 1 post-baseline scan (n=18). The bar colors indicate the type of therapy received immediately preceding study entry. The numerical values presented with each bar indicate the number of lines of prior treatment. *per Investigator Assessment PR* 4 (20%) SD 7 (35%) Not Evaluable 2 (10%) DCR 11 (55%) RECIST 1.1 ORR = 15% (95% CI, 3.2 - 37.9%) Figure 9: Duration of TreatmentSwimmer plot showing time on study and time of disease progression (n=20). mPFS: NR vs. 2.2 monthsHR 0.19 (95% CI, 0.03-1.09)p = 0.06 Figure 10: Progression Free Survival – Injection Site Reactions (ISR)KM plot of patient progression free survival (n=20) with ISR subgroups (yes or no) shows progression-free survival benefit in patients experiencing at least one injection site reaction to HS-110 during study treatment. Median PFS: 2.7 months(95% CI, 1.8; 4.0) Median OS = Not Reached(95% CI, 8.1 months – NR) Figure 11: Immune Activity and SurvivalPeripheral blood obtained before and during treatment (Weeks 4, 7, 13, EOT; n=24) to measure HS-110 derived immune reactivity. ELISPOTs represent INF-Ɣ secretion from T cells in culture after stimulation with HS-110 lysate. High = Patients with absolute ELISPOT increases above the group median; Low = Patients with absolute ELISPOT increases below the group median. Figure 12: Changes in the Tumor MicroenvironmentUsing AQUA (CD3, Ki67, Granzyme B, PD-1, PD-L1) and InForm (CD4, Foxp3) immunohistochemistry-based analysis, cell phenotypes were quantitated in the tumor microenvironment (TME) of a patient at baseline and after 10 weeks of combination treatment. Primary EndpointsPh 1b: SafetyPh 2: ORR (RECIST 1.1)Secondary EndpointsOS, PFS, DCR, DOR and iRECIST measurementsExploratory EndpointsImmune response, and correlation of clinical outcomes with Baseline CD8+ TIL levels, and PD-L1 expression on tumor cells. Figure 2: Study DesignThis single-arm, open-label trial has a phase 1b portion of 15 previously treated patients that have never received a CPI with a phase 2 expansion, that added Cohort B: Patients with PD after treatment failure with CPI. Adverse Events Adverse events (AEs) occurring in >10% of patients in the safety population (n=75) are: fatigue (31%), cough (21%), diarrhea (15%), anemia (13%), dyspnea (13%), nausea (13%), pruritis (13%), arthralgia (12%), hypoalbuminemia (12%), decreased appetite (11%), hyponatremia (11%), dizziness (11%) and constipation (11%). There were two grade 5 AEs, pulmonary embolism and acute myocardial infarction, neither of which were deemed related to treatment. On study Disease Progression Alive Deceased Figure 5b: Changes in Tumor Burden from Baseline Spider plot of tumor kinetics (n=42) demonstrating durability of decreased burden. For study-related correspondence, contact lmcdermott@heatbio.com