CORPORATE PRESENTATION
Published on May 29, 2020
EXHIBIT 99.4

Heat Biologics NASDAQ: HTBX CORPORATE PRESENTATION MAY 2020 1

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, autoimmune diseases and infectious diseases , our planned discovery and development of a COVID - 19 vaccine, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 2019 , our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent filings with the Securities and Exchange Commission (collectively, our “SEC Filings”) . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2
Snapshot of Heat Biologics (Nasdaq: HTBX) • US - based biopharmaceutical company developing potential first - in - class immunotherapy products • HS - 110, an “off - the - shelf” cell - based immunotherapy product that has the potential to improve PD(L) - 1 therapy - Ongoing Phase 2 program demonstrates signals of efficacy in PD(L) - 1 progressor and PD(L) - 1 naïve patients • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signals - IND clearance by US FDA, Phase 1 initiated • COVID - 19 vaccine program aims to engineer multiple viral protein regions into our gp96 platform - Target to generate long - term innate and adaptive immune responses. Currently in preclinical development • PTX - 35 for T - cell activation and co - stimulation - IND clearance and first patient dosing expected by end of Q2 2020 - Preclinical synergy with anti - PD - (L)1 when combined with antigen - driven immunotherapies • Experienced management team with proven track record advancing oncology drugs to the market 3
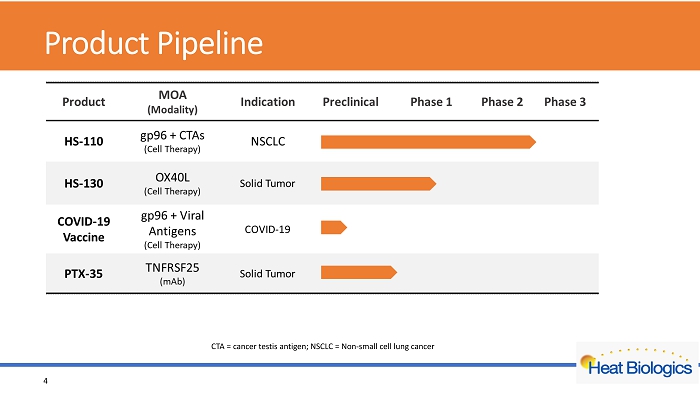
Product Pipeline 4 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer
HS - 110 Overview • HS - 110 is a Phase 2 cell - based immunotherapy administered in combination with PD - (L)1 therapies to improve clinical outcomes for NSCLC patients - Allogeneic cells with engineered gp96 to present multiple cancer testis antigens - Selectively activate CD8+ “killer” T cells - gp96 can up - regulate T - cell co - stimulation and maturation of antigen presenting cells (APCs) • PD - (L)1 is approved for multiple cancers and combination approaches may enhance survival benefits • Combination of HS - 110 and PD - (L)1 therapy may benefit patients in multiple treatment settings 5 References: Strbo et al 2013. Immunologic research; Strbo et al 2013. Journal of immunology; Yifei Wang et al. 2018. J Immunol; Heat Biologics Internal data; ‡ Shukuya & Carbone 2016. Journal of Thoracic Oncology, Vol.11 No.7: 976 - 988
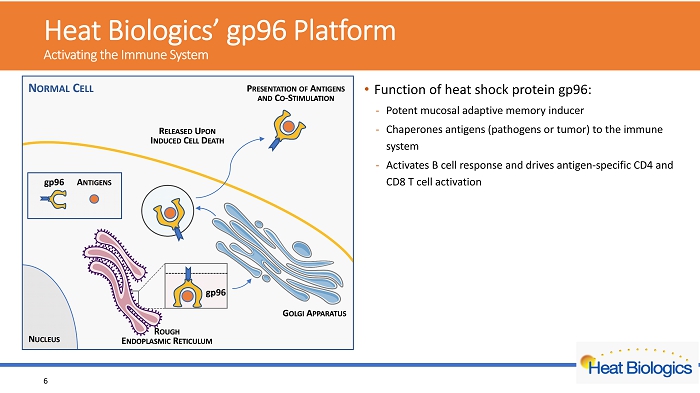
H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4 and CD8 T cel l activation • platform • Leverages gp96’s role as a natural molecular warning system • Engineered to secrete viral antigens bound to gp96 • Off - the - shelf allogeneic cell vaccine • Feasible for large scale manufacturing • Amenable to stockpiling • Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 6
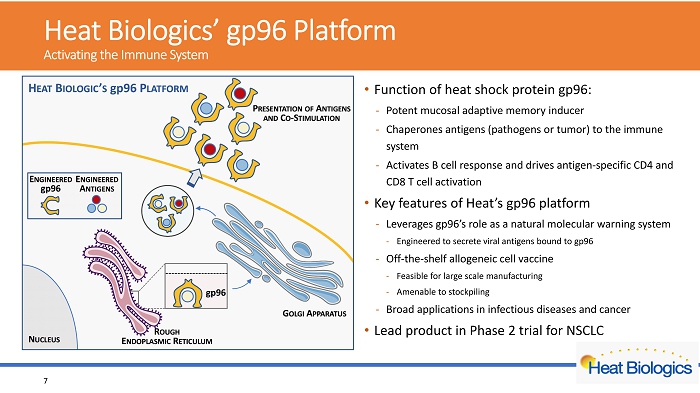
H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4 and CD8 T cel l activation • Key features of Heat’s gp96 platform - Leverages gp96’s role as a natural molecular warning system - Engineered to secrete viral antigens bound to gp96 - Off - the - shelf allogeneic cell vaccine - Feasible for large scale manufacturing - Amenable to stockpiling - Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 7
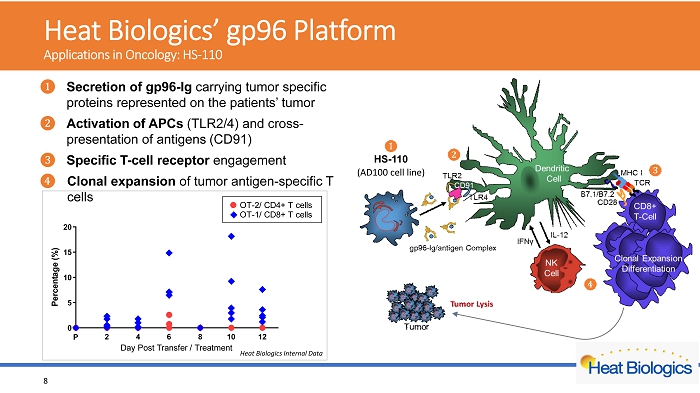
H eat Biologics’ gp96 Platform Applications in Oncology: HS - 110 2 4 6 8 10 12 0 5 10 15 20 Day Post Transfer / Treatment P e r c e n t a g e ( % ) P OT-2/ CD4+ T cells OT-1/ CD8+ T cells Heat Biologics Internal Data 8 ᬚ Secretion of gp96 - Ig carrying tumor specific proteins represented on the patients’ tumor ᬛ Activation of APCs (TLR2/4) and cross - presentation of antigens (CD91) ᬜ Specific T - cell receptor engagement ᬝ Clonal expansion of tumor antigen - s pecific T cells HS - 110 (AD100 cell line)
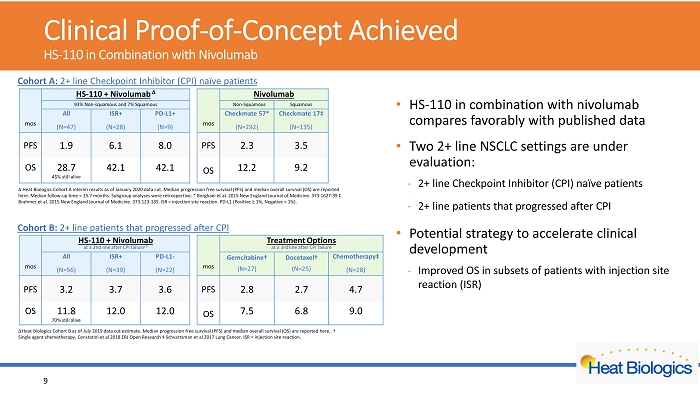
Cohort A: 2+ line Checkpoint Inhibitor (CPI) naïve patients Cohort B: 2+ line patients that progressed after CPI Δ Heat Biologics Cohort A interim results as of January 2020 data cut. Median progression free survival (PFS) and median overal l s urvival (OS) are reported here. Median follow - up time = 15.7 months. Subgroup analyses were retrospective. * Borghaei et al. 2015 New England Journal of Medicine. 373:1627 - 39 ‡ Brahmer et al. 2015 New England Journal of Medicine. 373:123 - 135. ISR = injection site reaction. PD - L1 (Positive ≥ 1%, Negative < 1%). Δ Heat Biologics Cohort B as of July 2019 data cut estimate. Median progression free survival (PFS) and median overall survival (O S) are reported here.. † Single agent chemotherapy, C onstatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer. ISR = injection site reaction. Clinical Proof - of - Concept Achieved HS - 110 in Combination with Nivolumab • HS - 110 in combination with nivolumab compares favorably with published data • Two 2+ line NSCLC settings are under evaluation: - 2+ line Checkpoint Inhibitor (CPI) naïve patients - 2+ line patients that progressed after CPI • Potential strategy to accelerate clinical development - Improved OS in subsets of patients with injection site reaction (ISR) 9
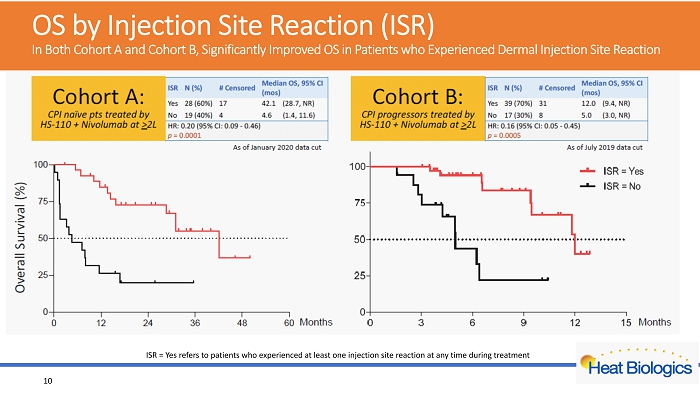
OS by Injection Site Reaction (ISR) In Both Cohort A and Cohort B, Significantly Improved OS in Patients who Experienced Dermal Injection Site Reaction ISR = Yes refers to patients who experienced at least one injection site reaction at any time during treatment 10
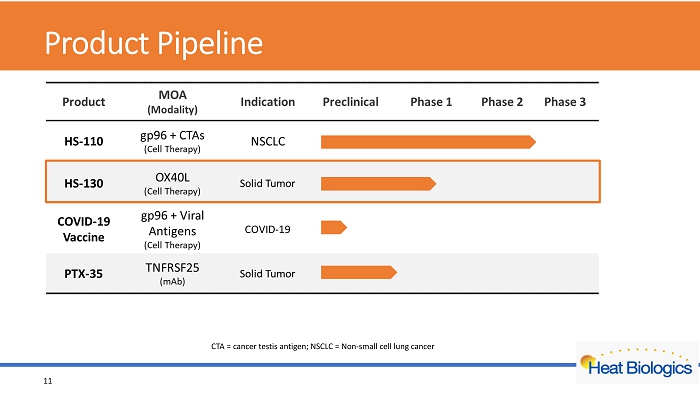
Product Pipeline 11 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer

HS - 130 Overview • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signal - Leverage HS - 110 clinical experience and manufacturing know - how - Addition of OX40L fusion protein to extend and expand T cell memory • Mechanism of action offers broad market potential • Phase 1 currently enrolling • Heat Biologics has worldwide rights 12
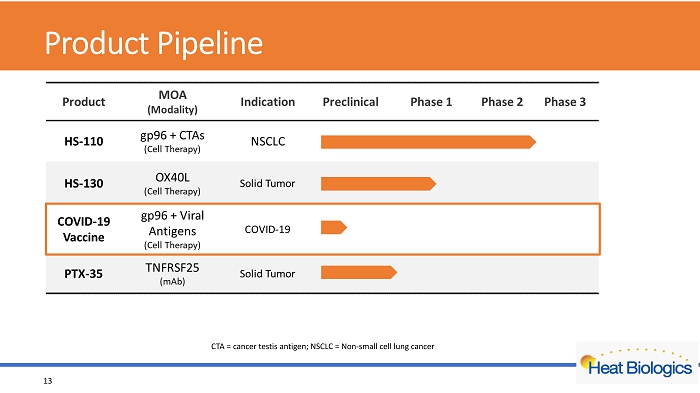
Product Pipeline 13 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer
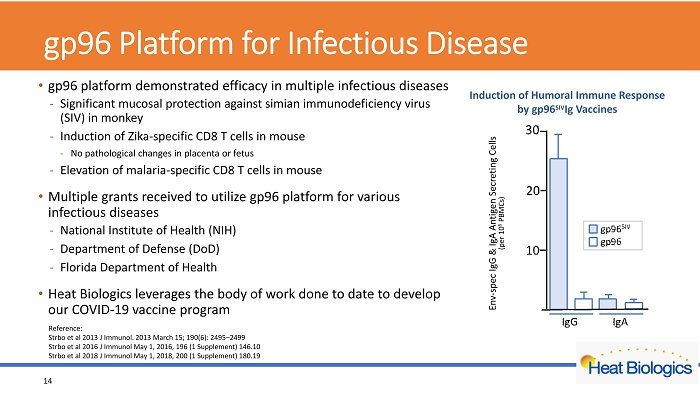
gp96 Platform for Infectious Disease • gp96 platform demonstrated efficacy in multiple infectious diseases - Significant mucosal protection against simian immunodeficiency virus (SIV) in monkey - Induction of Zika - specific CD8 T cells in mouse - No pathological changes in placenta or fetus - Elevation of malaria - specific CD8 T cells in mouse • Multiple grants received to utilize gp96 platform for various infectious diseases - National Institute of Health (NIH) - Department of Defense (DoD) - Florida Department of Health • Heat Biologics leverages the body of work done to date to develop our COVID - 19 vaccine program 14 Reference: Strbo et al 2013 J Immunol. 2013 March 15; 190(6): 2495 – 2499 Strbo et al 2016 J Immunol May 1, 2016, 196 (1 Supplement) 146.10 Strbo et al 2018 J Immunol May 1, 2018, 200 (1 Supplement) 180.19 Env - spec IgG & IgA Antigen Secreting Cells (per 10 6 PBMCs) Induction of Humoral Immune Response by gp96 SIV Ig Vaccines
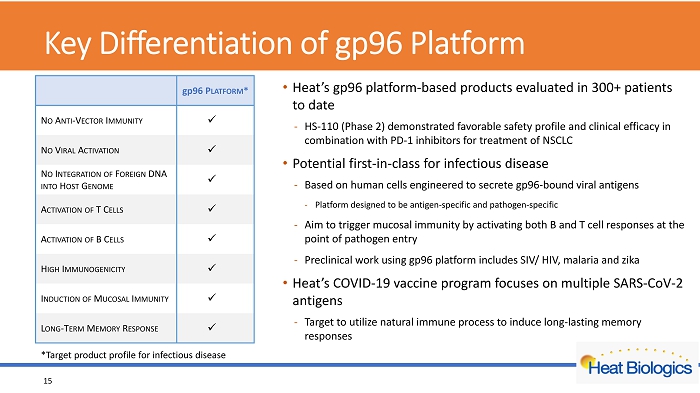
Key Differentiation of gp96 Platform • Heat’s gp96 platform - based products evaluated in 300+ patients to date - HS - 110 (Phase 2) demonstrated favorable safety profile and clinical efficacy in combination with PD - 1 inhibitors for treatment of NSCLC • Potential first - in - class for infectious disease - Based on human cells engine ered to secrete gp96 - bound viral antigens - Platform designed to be antigen - specific and pathogen - specific - Aim to trigger mucosal immunity by activating both B and T cell responses at the point of pathogen entry - Preclinical work using gp96 platform includes SIV/ HIV, malaria and zika • Heat’s COVID - 19 vaccine program focuses on multiple SARS - CoV - 2 antigens - Target to utilize natural immune process to induce long - lasting memory responses 15 gp 96 P LATFORM * N O A NTI - V ECTOR I MMUNITY x N O V IRAL A CTIVATION x N O I NTEGRATION OF F OREIGN DNA INTO H OST G ENOME x A CTIVATION O F T C ELLS x A CTIVATION OF B C ELLS x H IGH I MMUNOGENICITY x I NDUCTION OF M UCOSAL I MMUNITY x L ONG - T ERM M EMORY R ESPONSE x *Target product profile for infectious disease
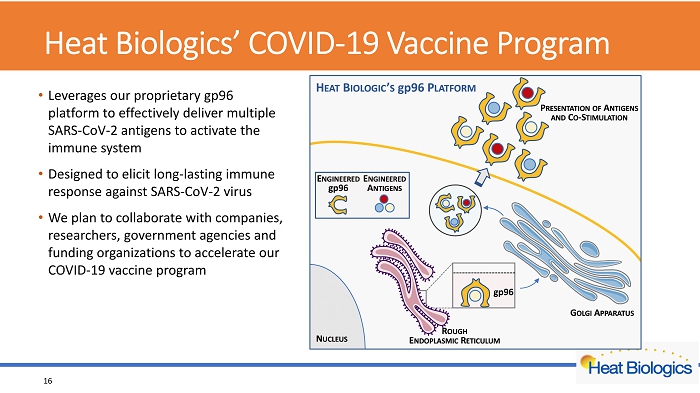
Heat Biologics’ COVID - 19 Vaccine Program • Leverages our proprietary gp96 platform to effectively deliver multiple SARS - CoV - 2 antigens to activate the immune system • Designed to elicit long - lasting immune response against SARS - CoV - 2 virus • We plan to collaborate with companies, researchers, government agencies and funding organizations to accelerate our COVID - 19 vaccine program 16
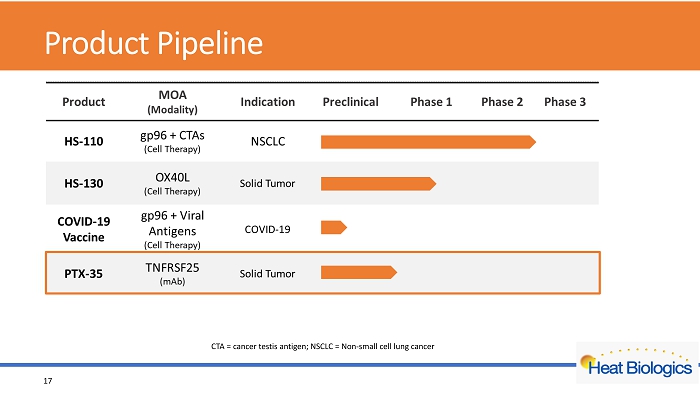
Product Pipeline 17 CTA = cancer testis antigen; NSCLC = Non - small cell lung cancer
PTX - 35 Overview • Potential first - in - class T cell co - stimulator targeting TNFRSF25, with preferential specificity to generate “memory” CD8+ T cells - IND clearance and first patient dosing expected by end of Q2 2020 • Broad market potential - Efficacy demonstrated in multiple preclinical in vivo colon, lung and breast cancer models • Synergistic combination with immunotherapies including HS - 110 and checkpoint inhibitors • Awarded a $15.2M grant to fund 70 patients clinical trial • Worldwide rights licensed by Heat Biologics 18
Snapshot of Heat Biologics (Nasdaq: HTBX) • US - based biopharmaceutical company developing potential first - in - class immunotherapy products • HS - 110, an “off - the - shelf” cell - based immunotherapy product that has the potential to improve PD(L) - 1 therapy - Ongoing Phase 2 program demonstrates signals of efficacy in PD(L) - 1 progressor and PD(L) - 1 naïve patients • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signals - IND clearance by US FDA, Phase 1 initiated • COVID - 19 vaccine program aims to engineer multiple viral protein regions into our gp96 platform - Target to generate long - term innate and adaptive immune responses. Currently in preclinical development • PTX - 35 for T - cell activation and co - stimulation - IND clearance and first patient dosing expected by end of Q2 2020 - Preclinical synergy with anti - PD - (L)1 when combined with antigen - driven immunotherapies • Experienced management team with proven track record advancing oncology drugs to the market 19

Heat Biologics NASDAQ: HTBX 20