POWER POINT PRESENTATION
Published on January 27, 2016
EXHIBIT 99.1

Phacilitate Immunotherapy World Conference January 26, 2016

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2
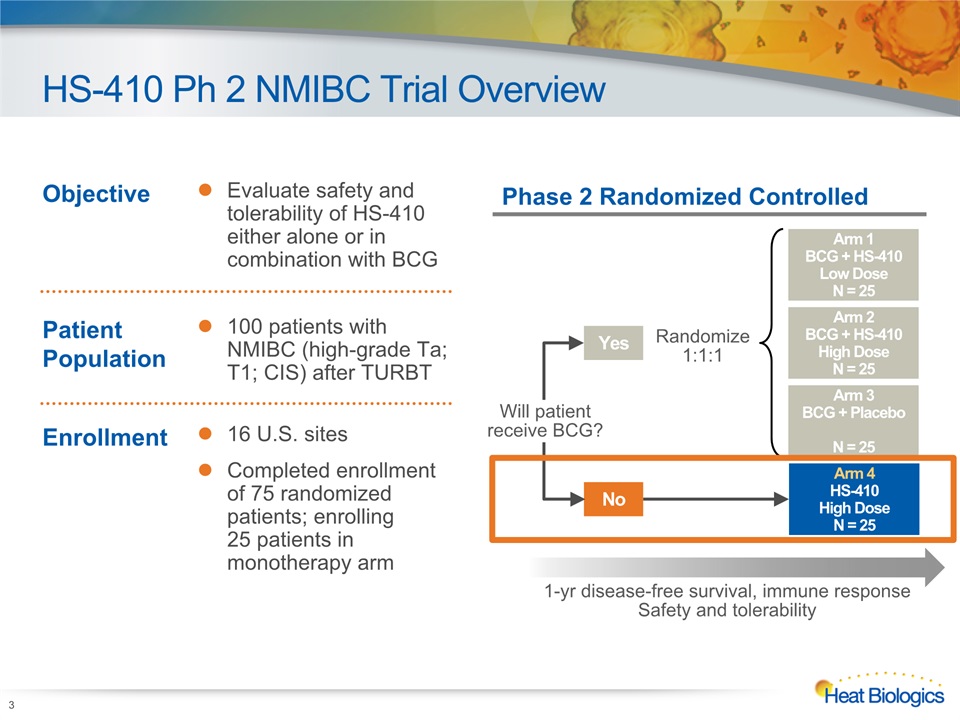
HS-410 Ph 2 NMIBC Trial Overview 3 Objective Evaluate safety and tolerability of HS-410 either alone or in combination with BCG Patient Population 100 patients with NMIBC (high-grade Ta; T1; CIS) after TURBT Enrollment 16 U.S. sitesCompleted enrollment of 75 randomized patients; enrolling 25 patients in monotherapy arm Phase 2 Randomized Controlled Arm 2BCG + HS-410High DoseN = 25 Arm 1BCG + HS-410Low DoseN = 25 Arm 3BCG + PlaceboN = 25 Arm 4HS-410 High DoseN = 25 Yes Randomize1:1:1 No Will patientreceive BCG? 1-yr disease-free survival, immune responseSafety and tolerability
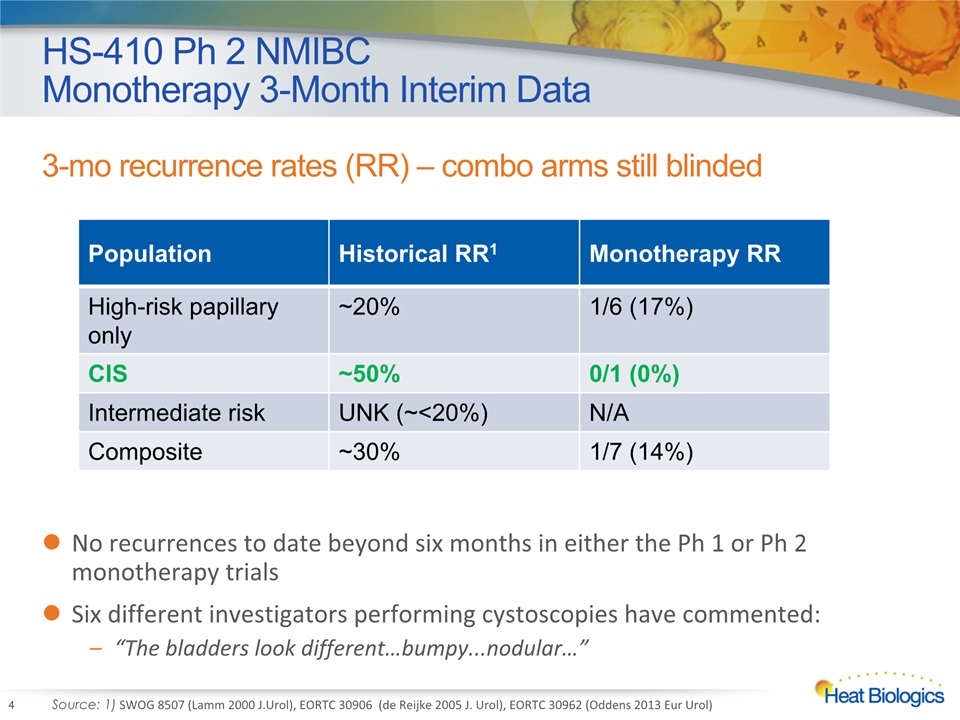
HS-410 Ph 2 NMIBC Monotherapy 3-Month Interim Data 3-mo recurrence rates (RR) – combo arms still blinded 4 Population Historical RR1 Monotherapy RR High-risk papillary only ~20% 1/6 (17%) CIS ~50% 0/1 (0%) Intermediate risk UNK (~<20%) N/A Composite ~30% 1/7 (14%) Source: 1) SWOG 8507 (Lamm 2000 J.Urol), EORTC 30906 (de Reijke 2005 J. Urol), EORTC 30962 (Oddens 2013 Eur Urol) No recurrences to date beyond six months in either the Ph 1 or Ph 2 monotherapy trialsSix different investigators performing cystoscopies have commented:“The bladders look different…bumpy...nodular…”
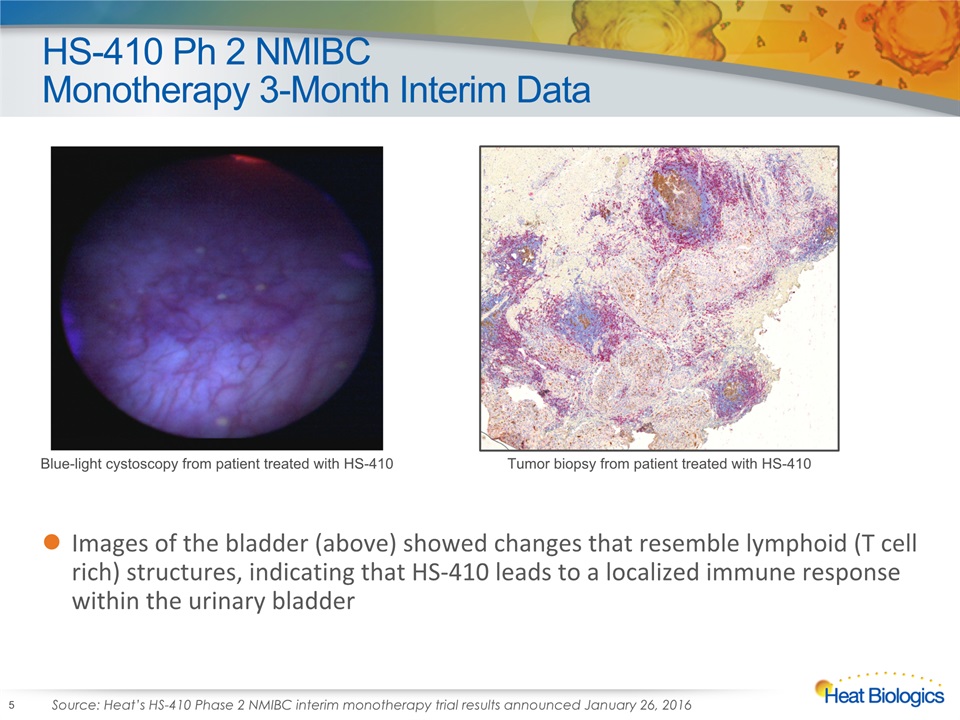
HS-410 Ph 2 NMIBC Monotherapy 3-Month Interim Data Images of the bladder (above) showed changes that resemble lymphoid (T cell rich) structures, indicating that HS-410 leads to a localized immune response within the urinary bladder 5 Blue-light cystoscopy from patient treated with HS-410 Tumor biopsy from patient treated with HS-410 Source: Heat’s HS-410 Phase 2 NMIBC interim monotherapy trial results announced January 26, 2016